Soil Pests – Cassava and Other Crops
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hemiptera- Heteroptera) En México, Con Un Listado De Las Especies Conocidas Anales Del Instituto De Biología
Anales del Instituto de Biología. Serie Zoología ISSN: 0368-8720 [email protected] Universidad Nacional Autónoma de México México Mayorga MARTÍNEZ, Ma. Cristina Revisión genérica de la familia Cydnidae (Hemiptera- Heteroptera) en México, con un listado de las especies conocidas Anales del Instituto de Biología. Serie Zoología, vol. 73, núm. 2, julio-diciembre, 2002, pp. 157-192 Universidad Nacional Autónoma de México Distrito Federal, México Disponible en: http://www.redalyc.org/articulo.oa?id=45873203 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología 73(2): 157-192. 2002 Revisión genérica de la familia Cydnidae (Hemiptera- Heteroptera) en México, con un listado de las especies conocidas MA. CRISTINA MAYORGA MARTÍNEZ* Resumen. Se revisa la familia Cydnidae (Hemiptera-Heteroptera) para México, representada por 12 géneros: Amnestus Dallas, Cyrtomenus Amyot & Serville, Dallasiellus Berg, Ectinopus Dallas, Melanaethus Uhler, Microporus Uhler, Pangaeus Stål, Prolobodes Amyot & Serville, Rhytidoporus Uhler, Tominotus Mulsant & Rey, Scaptocoris Perty, Sehirus Amyot & Serville, pertenecientes a cuatro subfamilias: Amnestinae, Cydninae Scaptocorinae, Sehirinae; se incluyen datos de distribución de cada -

2020 Olympic Games Statistics
2020 Olympic Games Statistics - Women’s 400m by K Ken Nakamura The records to look for in Tokyo: 1) Can Miller-Uibo become only the second (after Perec) 400m sprinter to win the Olympic twice. Summary Page: All time Performance List at the Olympic Games Performance Performer Time Name Nat Pos Venue Year 1 1 48.25 Marie -Jose Perec FRA 1 Atlanta 1996 2 2 48.63 Cathy Freeman AUS 2 Atla nta 1996 3 3 48.65 Olga Bryzgina URS 1 Seoul 1988 4 4 48.83 Valerie Brisco -Hooks USA 1 Los Angeles 1984 4 48 .83 Marie Jose -Perec 1 Barcelona 1992 6 5 48.88 Marita Koch GDR 1 Moskva 1980 7 6 49.05 Chandra Cheeseborough USA 2 Los Angeles 1984 Slowest winning time since 1976: 49.62 by Christine Ohuruogu (GBR) in 2008 Margin of Victory Difference Winning time Name Nat Venue Year Max 1.23 49.28 Irena Szewinska POL Montreal 1976 Min 0.07 49.62 Christine Ohuruogu GBR Beijing 20 08 49.44 Shaunae Miller BAH Rio de Janeiro 2016 Fastest time in each round Round Time Name Nat Venue Year Final 48.25 Marie -Jose Perec FRA Atlanta 1996 Semi-final 49.11 Olga Nazarova URS Seoul 1988 First round 50.11 Sanya Richards USA Athinai 2004 Fastest non-qualifier for the final Time Position Name Nat Venue Year 49.91 5sf1 Jillian Richardson CAN Seoul 1988 Best Marks for Places in the Olympics Pos Time Name Nat Venue Year 1 48.25 Marie -Jose Perec FRA Atlanta 1996 2 48.63 Cathy Freeman AUS Atlanta 1996 3 49.10 Falilat Ogunkoya NGR Atlanta 1996 Last nine Olympics: Year Gold Nat Time Silver Nat Time Bronze Nat Time 2016 Shaunae Miller BAH 49.44 Allyson Felix USA 49.51 Shericka Jackson -

Libro ING CAC1-36:Maquetación 1.Qxd
© Enrique Montesinos, 2013 © Sobre la presente edición: Organización Deportiva Centroamericana y del Caribe (Odecabe) Edición y diseño general: Enrique Montesinos Diseño de cubierta: Jorge Reyes Reyes Composición y diseño computadorizado: Gerardo Daumont y Yoel A. Tejeda Pérez Textos en inglés: Servicios Especializados de Traducción e Interpretación del Deporte (Setidep), INDER, Cuba Fotos: Reproducidas de las fuentes bibliográficas, Periódico Granma, Fernando Neris. Los elementos que componen este volumen pueden ser reproducidos de forma parcial siem- pre que se haga mención de su fuente de origen. Se agradece cualquier contribución encaminada a completar los datos aquí recogidos, o a la rectificación de alguno de ellos. Diríjala al correo [email protected] ÍNDICE / INDEX PRESENTACIÓN/ 1978: Medellín, Colombia / 77 FEATURING/ VII 1982: La Habana, Cuba / 83 1986: Santiago de los Caballeros, A MANERA DE PRÓLOGO / República Dominicana / 89 AS A PROLOGUE / IX 1990: Ciudad México, México / 95 1993: Ponce, Puerto Rico / 101 INTRODUCCIÓN / 1998: Maracaibo, Venezuela / 107 INTRODUCTION / XI 2002: San Salvador, El Salvador / 113 2006: Cartagena de Indias, I PARTE: ANTECEDENTES Colombia / 119 Y DESARROLLO / 2010: Mayagüez, Puerto Rico / 125 I PART: BACKGROUNG AND DEVELOPMENT / 1 II PARTE: LOS GANADORES DE MEDALLAS / Pasos iniciales / Initial steps / 1 II PART: THE MEDALS WINNERS 1926: La primera cita / / 131 1926: The first rendezvous / 5 1930: La Habana, Cuba / 11 Por deportes y pruebas / 132 1935: San Salvador, Atletismo / Athletics -

Code-Switching En La Novela Latino-Estadounidense De Diáspora: La Recreación Del Territorio Lingüístico
CODE-SWITCHING EN LA NOVELA LATINO-ESTADOUNIDENSE DE DIÁSPORA: LA RECREACIÓN DEL TERRITORIO LINGÜÍSTICO MARÍA XIMENA RESTREPO HURTADO UNIVERSIDAD PONTIFICIA BOLIVARIANA ESCUELA DE TEOLOGÍA, FILOSOFÍA Y HUMANIDADES ESTUDIOS LITERARIOS MEDELLÍN 2015 CODE-SWITCHING EN LA NOVELA LATINO-ESTADOUNIDENSE DE DIÁSPORA: LA RECREACIÓN DEL TERRITORIO LINGÜÍSTICO MARÍA XIMENA RESTREPO HURTADO Trabajo de grado para optar al título de Profesional en Estudios Literarios Asesor JORGE ENRIQUE CUÉLLAR AGUDELO Profesional en Estudios Literarios MG en Educación UNIVERSIDAD PONTIFICIA BOLIVARIANA ESCUELA DE TEOLOGÍA, FILOSOFÍA Y HUMANIDADES ESTUDIOS LITERARIOS MEDELLÍN 2015 Nota de aceptación ____________________________ ____________________________ ____________________________ ____________________________ ____________________________ Firma Nombre: Presidente del jurado _____________________________________ Firma Nombre: Jurado _____________________________________ Firma Nombre: Jurado Medellín, Agosto 06 de 2015 (Agosto 4, 2015) María Ximena Restrepo Hurtado Declaro que este trabajo de grado no ha sido presentado para optar a un título, ya sea en igual forma o con variaciones, en esta o cualquier otra universidad” Art 82 Régimen Discente de Formación Avanzada. Firma A Sophia, a mi madre y mi padre. Al viaje y al retorno. AGRADECIMIENTOS A Paula Dejanon, por todo. A todos los que me regalaron libros para mi tesis: mis padres, mi tío Gustavo, mis amigos, compañeros, profesores. A Jorge Cuéllar, siempre paciente, siempre emocionado por mis logros. A María Clemencia Sánchez: gracias por Adichie, por las heterotopías y los naufragios, por todo lo que llevó a este trabajo. A todos los que me recomendaron textos y me mostraron interés por el tema, a Laura Correa. A Gustavo Pérez Firmat, quien cuando le escribí, una desconocida desde Colombia, me respondió, me aconsejó y me ayudó. -
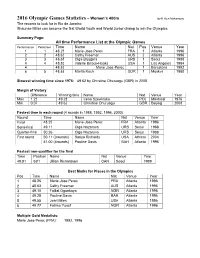
2016 Olympic Games Statistics
2016 Olympic Games Statistics - Women’s 400m by K Ken Nakamura The records to look for in Rio de Janeiro: Shaunae Miller can become the first World Youth and World Junior champ to win the Olympics. Summary Page: All time Performance List at the Olympic Games Performance Performer Time Name Nat Pos Venue Year 1 1 48.25 Marie -Jose Perec FRA 1 Atlanta 1996 2 2 48.63 Cathy Freeman AUS 2 Atlanta 1996 3 3 48.65 Olga Bryzgina URS 1 Seoul 1988 4 4 48.83 Valer ie Brisco -Hooks USA 1 Los Angeles 1984 4 48.83 Marie Jose -Perec 1 Barcelona 1992 6 5 48.88 Marita Koch GDR 1 Moskva 1980 Slowest winning time since 1976: 49.62 by Christine Ohuruogu (GBR) in 2008 Margin of Victory Difference Winning time Name Nat Venue Year Max 1.23 49.28 Irena Szewinska POL Montreal 1976 Min 0.07 49.62 Christine Ohuruogu GBR Beijing 2008 Fastest time in each round (4 rounds in 1988, 1992, 1996, 2000) Round Time Name Nat Venue Year Final 48.25 Marie -Jose Perec FRA Atlant a 1996 Semi-final 49.11 Olga Nazarova URS Seoul 1988 Quarter-final 50.26 Olga Nazarova URS Seoul 1988 First round 50.11 (3rounds) Sanya Richards USA Athinai 2004 51.00 (4rounds) Pauline Davis BAH Atlanta 1996 Fastest non-qualifier for the final Time Position Name Nat Venue Year 49.91 5sf1 Jillian Richardson CAN Seoul 1988 Best Marks for Places in the Olympics Pos Time Name Nat Venue Year 1 48.25 Marie -Jose Perec FRA Atlanta 1996 2 48.63 Cathy Freeman AUS Atlanta 1996 3 49.10 Falilat O gunkoya NGR Atlanta 1996 4 49.28 Pauline Davis BAH Atlanta 1996 5 49.55 Jearl Miles USA Atlanta 1996 6 49.77 Fatima Yusuf -

Resultados Troféu Brasil 2021
XL Troféu Brasil Loterias Caixa de Atletismo São Paulo - SP Data : 10/06/2021 Hora : 13:15 Resultado Oficial - 1ª Etapa Categoria: Adulto 400 metros - Feminino - Semifinal Recorde Nome Clube Local Data CR 51.08 Maria Magnolia Souza F UE Funilen São José Rio Pre 22/06/199 NR 50.62 Maria Magnólia Souza F RN Rovereto, ITA 21/08/199 AR 49.64 Ximena Restrepo COL Barcelona, ESP 05/08/199 Horário: Umidade: Temperatura: 1ª Série Início: 13:15 74 % 22 ºC Col. No. CBAt Atleta Nasc. UF Clube Reac Marca Q 1 663 63208 Tiffani Beatriz Domingos Silva do Na 06/05/99 SP ORCAMPI 0.252 53.22 Q 2 457 55219 Rita de Cassia Ferreira Silva 10/05/99 SP ASPMP 0.260 54.63 Q PB 3 660 59117 Marlene Ewellyn Silva dos Santos 24/10/99 SP ORCAMPI 0.306 55.45 4 446 55708 Jacqueline Cristiane da Silva Alves 18/02/98 SP AAARP 0.541 55.90 5 80 16600 Joelma das Neves Sousa 13/07/84 MA CT - Maranhão 0.201 56.58 6 775 60564 Ester dos Santos Moura 15/03/00 DF CORGAMA 0.253 58.55 SB 7 778 65599 Marina Severina Pereira de Siqueir 01/04/03 DF CASO 0.232 59.82 Horário: Umidade: Temperatura: 2ª Série Início: 13:22 74 % 22 ºC Col. No. CBAt Atleta Nasc. UF Clube Reac Marca Q 1 177 48181 Tabata Vitorino de Carvalho 23/04/96 PR AA MARINGA 0.399 53.10 Q SB 2 310 27703 Liliane Cristina Barbosa Parrela 08/10/87 SC ACA 0.404 53.86 Q 3 836 64351 Giovana Rosalia dos Santos 08/08/00 SP APA - SP 0.276 54.68 q SB 4 539 49120 Daysiellen Atla Dias 24/03/97 SP E.C. -

Redalyc.SECONDARY METABOLITES of the ANNONACEAE, SOLANACEAE and MELIACEAE FAMILIES USED AS BIOLOGICAL CONTROL of INSECTS
Tropical and Subtropical Agroecosystems E-ISSN: 1870-0462 [email protected] Universidad Autónoma de Yucatán México Castillo-Sánchez, Luis Enrique; Jiménez-Osornio, Juan José; Delgado-Herrera, María América SECONDARY METABOLITES OF THE ANNONACEAE, SOLANACEAE AND MELIACEAE FAMILIES USED AS BIOLOGICAL CONTROL OF INSECTS Tropical and Subtropical Agroecosystems, vol. 12, núm. 3, septiembre-diciembre, 2010, pp. 445-462 Universidad Autónoma de Yucatán Mérida, Yucatán, México Available in: http://www.redalyc.org/articulo.oa?id=93915170004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Tropical and Subtropical Agroecosystems, 12 (2010): 445 -462 REVIEW [REVISIÓN] SECONDARY METABOLITES OF THE ANNONACEAE, SOLANACEAE Tropical and AND MELIACEAE FAMILIES USED AS BIOLOGICAL CONTROL OF INSECTS Subtropical [METABOLITOS SECUNDARIOS DE LAS FAMILIAS ANNONACEAE, SOLANACEAE Y MELIACEAE USADAS COMO CONTROL BIOLÓGICO Agroecosystems DE INSECTOS] Luis Enrique Castillo-Sánchez1*, Juan José Jiménez-Osornio2 and María América Delgado-Herrera3. 1Technological Institute of Tizimin 3.5 km final highway Cupul airport to Tizimin. Tizimin, Yucatan, Mexico. Email: [email protected] 2Tropical Natural Resources Management and Conservation Department, Biological Sciences and Animal Husbandry Campus, Autonomous University of Yucatan. -

Insect Pathogens As Biological Control Agents: Back to the Future ⇑ L.A
Journal of Invertebrate Pathology 132 (2015) 1–41 Contents lists available at ScienceDirect Journal of Invertebrate Pathology journal homepage: www.elsevier.com/locate/jip Insect pathogens as biological control agents: Back to the future ⇑ L.A. Lacey a, , D. Grzywacz b, D.I. Shapiro-Ilan c, R. Frutos d, M. Brownbridge e, M.S. Goettel f a IP Consulting International, Yakima, WA, USA b Agriculture Health and Environment Department, Natural Resources Institute, University of Greenwich, Chatham Maritime, Kent ME4 4TB, UK c U.S. Department of Agriculture, Agricultural Research Service, 21 Dunbar Rd., Byron, GA 31008, USA d University of Montpellier 2, UMR 5236 Centre d’Etudes des agents Pathogènes et Biotechnologies pour la Santé (CPBS), UM1-UM2-CNRS, 1919 Route de Mendes, Montpellier, France e Vineland Research and Innovation Centre, 4890 Victoria Avenue North, Box 4000, Vineland Station, Ontario L0R 2E0, Canada f Agriculture and Agri-Food Canada, Lethbridge Research Centre, Lethbridge, Alberta, Canada1 article info abstract Article history: The development and use of entomopathogens as classical, conservation and augmentative biological Received 24 March 2015 control agents have included a number of successes and some setbacks in the past 15 years. In this forum Accepted 17 July 2015 paper we present current information on development, use and future directions of insect-specific Available online 27 July 2015 viruses, bacteria, fungi and nematodes as components of integrated pest management strategies for con- trol of arthropod pests of crops, forests, urban habitats, and insects of medical and veterinary importance. Keywords: Insect pathogenic viruses are a fruitful source of microbial control agents (MCAs), particularly for the con- Microbial control trol of lepidopteran pests. -

Dairy Alternative Development Colombia Program (LOL/DADCP)
Dairy Alternative Development Colombia Program (LOL/DADCP) USAID CA#514-A-00-03-00211-00 FINAL REPORT Submitted by Land O’Lakes, Inc. P.O. Box 64281 St. Paul, MN 55164-0281 USA Submitted to Camilo Sanchez Alternative Development Program USAID/Colombia Bogota, Colombia April 2006 © Copyright 2006 by Land O’Lakes, Inc. All rights reserved. Final Report Dairy Alternative Development Program Table of Contents 1. Executive Summary.................................................................................................................................. 2 2. Background.............................................................................................................................................. 5 3. Detailed Description of Activities......................................................................................................... 10 3.1 Technical Activities ......................................................................................................................... 10 3.1.1 Illicit Crop Elimination ............................................................................................................ 10 3.1.2 Licit Crop Establishment......................................................................................................... 12 3.1.3 Association Creation and/or Strengthening .......................................................................... 14 3.1.4 Milk Collection Center Establishment................................................................................... 15 3.1.5 Agro-Processor -

Mici) of the Inter-American Development Bank (Idb)
COMPLAINT BEFORE THE INDEPENDENT CONSULTATION AND INVESTIGATION MECHANISM (MICI) OF THE INTER-AMERICAN DEVELOPMENT BANK (IDB) Ituango Hydropower Plant (IHP), Antioquia, Colombia To: Victoria Márquez Mees Director, Independent Consultation and Investigation Mechanism Inter-American Development Bank 1300 New York Avenue, N.W. Washington, D.C. 20577 Email: [email protected] Tel.: 202-623-3952; Fax: 202-312-40 Re: Project 11794-04: EPM - IIC Ituango Hydropower Plant in Colombia http://www.iic.org/en/projects/colombia/11794-04/epm-ituango-hydropower-plant Filed by: Individuals, communities, and civil society organizations located in the municipalities of Ituango, Toledo, San Andrés de Cuerquia, Briceño, Valdivia, Sabanalarga, Peque, Caucasia, and Medellín, Antioquia, under the umbrella group Movimiento Ríos Vivos Antioquia. The following international organizations are providing support in this case: the Interamerican Association for Environmental Defense (AIDA), the Center for International Environmental Law (CIEL), and the International Accountability Project (IAP). Points of contact: ▪ Isabel Cristina Zuleta López ▪ Pedro Vicente With support from: Center for International Environmental Law (CIEL)1 Interamerican Association for Environmental Defense (AIDA)2 International Accountability Project (IAP)3 1 Point of contact: Carla García Zendejas 2 Point of contact 3 Point of contact: Alexandre Sampaio. 1 Antioquia, Colombia, June 5, 2018 Victoria Márquez Mees Director, Independent Consultation and Investigation Mechanism Inter-American Development Bank 1300 New York Avenue, N.W. Washington, D.C. 20577 Dear Ms. Márquez, We are writing to you as Colombian citizens and members of Movimiento Ríos Vivos Antioquía (MRV) on behalf of the communities that reside in the municipalities of Briceño, Ituango, Toledo, San Andrés de Cuerquia, Valdivia, Sabanalarga, Peque and Caucasia, Antioquia. -

The IAAF World Athletics Championships
SPORT PAGE | 03 PAGE | 07 Simona Halep’s NBA: Nets’ GM China woes expects Durant to continue with miss entire retirement 2019-20 season Thursday 26 September 2019 President re-elected by unanimous vote at IAAF Congress in Doha Coe wants sport to grow in his new term ARMSTRONG VAS cleaning up corruption in the sport. IAAF president THE PENINSULA “It’s been a tough four years, the first Sebastian Coe during two were the reforms -- the second two Sebastian Coe has been unanimously years were really making sure they were a press conference re-elected for a second term as President implemented,” he said. at the Sheraton This was not an easy of the International Association of “(We) got here pretty much imple- Grand Doha Resort journey. I genuinely am & Convention Hotel, Athletics Federations (IAAF). menting everything we said we’d do. very pleased and proud The 62-year-old, former Olympic and “I want the next four years to be the Doha, yesterday. world champion middle-distance runner, fun bit... I want the sport to grow,” he of the way the sport became head of world athletics’ governing added. has come together. Two body in 2015 and receives his second Yesterday’s Congress also saw the mandate just two days before the World election of four vice-presidents, and 13 hundred changes, the Athletics Championships open in Doha. members of the total 50. Athletics Integrity Unit Coe, who took over the reins during Ximena Restrepo, pole vault -- no sport has a unit in a period of turmoil in world athletics, was champion Sergey Bubka, Geoffrey re-elected by unanimous vote of the 203 Gardner and Nawaf Bin Mohammed Al the same space, it’s about delegates attending the International Saud were elected Vice-Presidents. -

Polyphagy in True Bugs: a Case Study of Leptoglossus Phyllopus (L.) (Hemiptera, Heteroptera, Coreidae)1
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Mitchell Paula Levin Artikel/Article: Polyphagy in True Bugs: A case study of Leptoglossus phyllopus (L.) (Hemiptera, Heteroptera, Coreidae) 1117-1134 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Polyphagy in True Bugs: A case study of Leptoglossus phyllopus (L.) (Hemiptera, Heteroptera, Coreidae)1 P.L. MITCHELL Abstract: The polyphagous species Leptoglossus phyllopus (L.) was examined with respect to host plant preference, tissue feeding specificity, seasonal dispersal among host plants, and life history. Mark-recap- ture, census, and rearing experiments demonstrated that this species exhibits true polyphagy, in that in- dividual bugs feed on plants from more than one family. Developmental parameters such as growth and survivorship did not differ among plants from several families, but did vary significantly with quality of host (e.g., wild vs. cultivated). Stadium duration, however, varied among wild host plant species in la- boratory experiments. Specialization on reproductive plant parts, coupled with sequential polyphagy and dispersal among a variety of seasonal host plants, allows multiple generations per year. Modes of fee- ding and preferred target tissues among coreids are discussed. Key words: leaffooted bug, Leptoglossus phyllopus, polyphagy, stylet sheath, target tissue. Introduction spp.), for example, employ a macerate-and- flush process, whereas an osmotic pump For phytophagous insects with piercing- mechanism is associated with coreids (MILES sucking mouthparts, feeding selectivity op- & TAYLOR 1994). However, some pentato- erates on two levels: preferred target tissue mids and lygaeids shift between salivary and host plant species.