Appendix Q – Core Guidelines for Determining Immediate Jeopardy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ISO Basic Latin Alphabet
ISO basic Latin alphabet The ISO basic Latin alphabet is a Latin-script alphabet and consists of two sets of 26 letters, codified in[1] various national and international standards and used widely in international communication. The two sets contain the following 26 letters each:[1][2] ISO basic Latin alphabet Uppercase Latin A B C D E F G H I J K L M N O P Q R S T U V W X Y Z alphabet Lowercase Latin a b c d e f g h i j k l m n o p q r s t u v w x y z alphabet Contents History Terminology Name for Unicode block that contains all letters Names for the two subsets Names for the letters Timeline for encoding standards Timeline for widely used computer codes supporting the alphabet Representation Usage Alphabets containing the same set of letters Column numbering See also References History By the 1960s it became apparent to thecomputer and telecommunications industries in the First World that a non-proprietary method of encoding characters was needed. The International Organization for Standardization (ISO) encapsulated the Latin script in their (ISO/IEC 646) 7-bit character-encoding standard. To achieve widespread acceptance, this encapsulation was based on popular usage. The standard was based on the already published American Standard Code for Information Interchange, better known as ASCII, which included in the character set the 26 × 2 letters of the English alphabet. Later standards issued by the ISO, for example ISO/IEC 8859 (8-bit character encoding) and ISO/IEC 10646 (Unicode Latin), have continued to define the 26 × 2 letters of the English alphabet as the basic Latin script with extensions to handle other letters in other languages.[1] Terminology Name for Unicode block that contains all letters The Unicode block that contains the alphabet is called "C0 Controls and Basic Latin". -

Threshold Digraphs
Volume 119 (2014) http://dx.doi.org/10.6028/jres.119.007 Journal of Research of the National Institute of Standards and Technology Threshold Digraphs Brian Cloteaux1, M. Drew LaMar2, Elizabeth Moseman1, and James Shook1 1National Institute of Standards and Technology, Gaithersburg, MD 20899 2The College of William and Mary, Williamsburg, VA 23185 [email protected] [email protected] [email protected] [email protected] A digraph whose degree sequence has a unique vertex labeled realization is called threshold. In this paper we present several characterizations of threshold digraphs and their degree sequences, and show these characterizations to be equivalent. Using this result, we obtain a new, short proof of the Fulkerson-Chen theorem on degree sequences of general digraphs. Key words: digraph realizations; Fulkerson-Chen theorem; threshold digraphs. Accepted: May 6, 2014 Published: May 20, 2014 http://dx.doi.org/10.6028/jres.119.007 1. Introduction Creating models of real-world networks, such as social and biological interactions, is a central task for understanding and measuring the behavior of these networks. A usual first step in this type of model creation is to construct a digraph with a given degree sequence. We examine the extreme case of digraph construction where for a given degree sequence there is exactly one digraph that can be created. What follows is a brief introduction to the notation used in the paper. For notation not otherwise defined, see Diestel [1]. We let G=( VE , ) be a digraph where E is a set of ordered pairs called arcs. If (,vw )∈ E, then we say w is an out-neighbor of v and v is an in-neighbor of w. -
![BBJ1RBR '(]~ F'o £.A2WB IJ.Boibiollb, •](https://docslib.b-cdn.net/cover/2472/bbj1rbr-fo-%C2%A3-a2wb-ij-boibiollb-442472.webp)
BBJ1RBR '(]~ F'o £.A2WB IJ.Boibiollb, •
801 80 mua1. , .. DDl&IOI o• OOLU•••• . I I:~ I BBJ1RBR '(]~ f'O £.A2WB IJ.BOIBIOllB, • •• • I r. • • Not Current- 1803 , 0 TIUI t ohn Tock r, q., of o ton, be i an k ep hi office at th eat of the o nbt practi , ither a an attorn y or a 0 &&all ontinue to b clerk of the ame. hat (until furth r orde ) it hall be om or cou ello to practi e in th· uch for thr e ea pa t in th uprem p cti ly b long, and that their pri ate r to be fair. &. t coun llors hall not pract• e a ........ ello , in h · court. 6. , That they hall re pecti el take the ------, do olemnly ear, that I ill or coun ellor of the court) uprightly, and upport the o titution of the nited &. t (unle and until it hall othe · e of · court hall be in the name of the That he cou ello and attorne , hall ake either an oath, or, in proper cribe b the rule of this court on that r•••, 1790, iz.: '' , , do olemnly be) that will demean m elf, as an attor .....· oo rt uprightl , an according to la , and that tion of he nited ta •'' hi f ju ice, in a er to the motion of the info him and the bar, that thi court of king' bench, and of chancery, in Eng ice of th• court; and that they will, tio therein circumstances may ren- x_...vu•• Not Current- 1803 0 '191, ebraary 4. -

Whenever There Is a Line Or Tilde Over a Vowel, It Is an Abbreviation for the Letters M Or N. in This Case, the Full Word Is Quadam
Whenever there is a line or tilde over a vowel, it is an abbreviation for the letters m or n. In this case, the full word is quadam. In this instance, the line over the e represents an omission of the letter n, giving the word debent when written in full. Whenever a word in Latin had an instance of the letter i in succession, the second i was always written as a j. This applies to all words wherein ii is present and also to the Roman numeral ii, which was written as ij. Therefore, cambij = cambii, negotijs = negotiis. The e with a cedilla was an abbreviation for the letters ae. This word spelled out is quae. Following the letter q, a period or semicolon was used as an abbreviation for the letters ue. The first example reads denique, the second one is quacunque. In the seventeenth century, the letter u and v were interchangeable and not yet viewed as two distinct letters. While not universal, a general rule is that if u or v began a word, it was written as a v; if a u or v occurred in the middle or end of a word, it was written as a u. In the 17th century, a lowercase s had two forms- one long and one short. As a general rule, when a lowercase s occurred as the first letter of the word or in any other place in the word except as the final letter, it was written with a long s, which resembles the letter f. -

Patterns Identification for Hitting Adjacent Key Errors Correction Using Neural Network Models
PATTERNS IDENTIFICATION FOR HITTING ADJACENT KEY ERRORS CORRECTION USING NEURAL NETWORK MODELS Jun Li Department of Land Economy, University of Cambridge, 19 Silver Street, Cambridge, U.K. Karim Ouazzane, Sajid Afzal, Hassan Kazemian Faculty of Computing, London Metropolitan University, London, U.K. Keywords: QWERTY Keyboard, Probabilistic Neural Network, Backpropagation, Key Distance, Time Gap, Error Margin Distance. Abstract: People with Parkinson diseases or motor disability miss-stroke keys. It appears that keyboard layout, key distance, time gap are affecting this group of people’s typing performance. This paper studies these features based on neural network learning algorithms to identify the typing patterns, further to correct the typing mistakes. A specific user typing performance, i.e. Hitting Adjacent Key Errors, is simulated to pilot this research. In this paper, a Time Gap and a Prediction using Time Gap model based on BackPropagation Neural Network, and a Distance, Angle and Time Gap model based on the use of Probabilistic Neural Network are developed respectively for this particular behaviour. Results demonstrate a high performance of the designed model, about 70% of all tests score above Basic Correction Rate, and simulation also shows a very unstable trend of user’s ‘Hitting Adjacent Key Errors’ behaviour with this specific datasets. 1 INTRODUCTION technologies to tackle the typing errors as a whole, while there is no specific solution and convincing Computer users with disabilities or some elderly results for solving the ‘Miss-stroke’ error. people may have difficulties in accurately In the field of computer science, a neural manipulating the QWERTY keyboard. For example, network is a mathematical model or computational motor disability can cause significant typing model that is inspired by the structure and/or mistakes. -

The Dot Product
The Dot Product In this section, we will now concentrate on the vector operation called the dot product. The dot product of two vectors will produce a scalar instead of a vector as in the other operations that we examined in the previous section. The dot product is equal to the sum of the product of the horizontal components and the product of the vertical components. If v = a1 i + b1 j and w = a2 i + b2 j are vectors then their dot product is given by: v · w = a1 a2 + b1 b2 Properties of the Dot Product If u, v, and w are vectors and c is a scalar then: u · v = v · u u · (v + w) = u · v + u · w 0 · v = 0 v · v = || v || 2 (cu) · v = c(u · v) = u · (cv) Example 1: If v = 5i + 2j and w = 3i – 7j then find v · w. Solution: v · w = a1 a2 + b1 b2 v · w = (5)(3) + (2)(-7) v · w = 15 – 14 v · w = 1 Example 2: If u = –i + 3j, v = 7i – 4j and w = 2i + j then find (3u) · (v + w). Solution: Find 3u 3u = 3(–i + 3j) 3u = –3i + 9j Find v + w v + w = (7i – 4j) + (2i + j) v + w = (7 + 2) i + (–4 + 1) j v + w = 9i – 3j Example 2 (Continued): Find the dot product between (3u) and (v + w) (3u) · (v + w) = (–3i + 9j) · (9i – 3j) (3u) · (v + w) = (–3)(9) + (9)(-3) (3u) · (v + w) = –27 – 27 (3u) · (v + w) = –54 An alternate formula for the dot product is available by using the angle between the two vectors. -

The Selnolig Package: Selective Suppression of Typographic Ligatures*
The selnolig package: Selective suppression of typographic ligatures* Mico Loretan† 2015/10/26 Abstract The selnolig package suppresses typographic ligatures selectively, i.e., based on predefined search patterns. The search patterns focus on ligatures deemed inappropriate because they span morpheme boundaries. For example, the word shelfful, which is mentioned in the TEXbook as a word for which the ff ligature might be inappropriate, is automatically typeset as shelfful rather than as shelfful. For English and German language documents, the selnolig package provides extensive rules for the selective suppression of so-called “common” ligatures. These comprise the ff, fi, fl, ffi, and ffl ligatures as well as the ft and fft ligatures. Other f-ligatures, such as fb, fh, fj and fk, are suppressed globally, while making exceptions for names and words of non-English/German origin, such as Kafka and fjord. For English language documents, the package further provides ligature suppression rules for a number of so-called “discretionary” or “rare” ligatures, such as ct, st, and sp. The selnolig package requires use of the LuaLATEX format provided by a recent TEX distribution, e.g., TEXLive 2013 and MiKTEX 2.9. Contents 1 Introduction ........................................... 1 2 I’m in a hurry! How do I start using this package? . 3 2.1 How do I load the selnolig package? . 3 2.2 Any hints on how to get started with LuaLATEX?...................... 4 2.3 Anything else I need to do or know? . 5 3 The selnolig package’s approach to breaking up ligatures . 6 3.1 Free, derivational, and inflectional morphemes . -
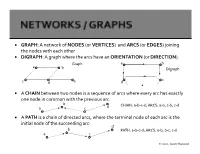
GRAPH: a Network of NODES (Or VERTICES) and ARCS (Or EDGES) Joining the Nodes with Each Other DIGRAPH: a Graph Where the Arcs Have an ORIENTATION (Or DIRECTION)
GRAPH: A network of NODES (or VERTICES) and ARCS (or EDGES) joining the nodes with each other DIGRAPH: A graph where the arcs have an ORIENTATION (or DIRECTION). Graph a b a b Digraph c c d e d A CHAIN between two nodes is a sequence of arcs where every arc has exactly one node in common with the previous arc b CHAIN: a-b-c-d; ARCS: a-b, c-b, c-d a c d A PATH is a chain of directed arcs, where the terminal node of each arc is the initial node of the succeeding arc: d b PATH: a-b-c-d; ARCS: a-b, b-c, c-d a c 2020, Jayant Rajgopal b d a c CYCLE: a-b-c-d; ARCS: a-b, c-b, c-d, d-a b d CIRCUIT: a-b-c-d; ARCS: a-b, b-c, c-d, d-a a c A graph is said to be CONNECTED if there is a continuous chain of edges joining any two vertices. A graph is STRONGLY CONNECTED if for all ordered pairs of vertices (i,j) there is a path of arcs from i to j. A graph is COMPLETE if every node is directly connected to every other node. c A TREE is a connected graph with no cycles. b d a A SPANNING TREE is a tree that contains all the nodes of a graph. b b e e a a c c Graph Spanning Tree d d 2020, Jayant Rajgopal GIVEN: A digraph G with a set of nodes 1,2,…,m, and a set of n directed arcs i-j emanating from node i and ending in node j, with each node i, a number bi that is the supply (if bi>0) or the demand (if bi<0); if bi=0, then the node is called a transshipment node, with each arc i-j, a flow of xij and a unit transportation / movement cost of cij. -

Approximations in Monte-Carlo Generators
Approximations in Monte-Carlo generators J. Bellm (Lund U.), Science Coffee, 25.1.2018 Colour Rearrangement for Dipole Showers Last friday: JB arXiv:1801.06113 J. Bellm (Lund U.), Science Coffee, 25.1.2018 Outline Walk though events generation What kind of approximations do we use? Can we improve the description? Recent developments from Lund J. Bellm (Lund U.), Science Coffee, 25.1.2018 What topic am I trying to cover? „…common words in the titles of the 2012 top 40 papers.“ symmetrymagazine.org J. Bellm (Lund U.), Science Coffee, 25.1.2018 What topic am I trying to cover? „…common words in the titles of the 2012 top 40 papers.“ symmetrymagazine.org J. Bellm (Lund U.), Science Coffee, 25.1.2018 What topic am I trying to cover? „…common words in the titles of the 2012 top 40 papers.“ symmetrymagazine.org J. Bellm (Lund U.), Science Coffee, 25.1.2018 How we simulate events? matrix elements event generators detector simulation analysis tools Rivet pdf J. Bellm (Lund U.), Science Coffee, 25.1.2018 How we simulate events? detector event generators matrix elements pdf picture: arXiv:1411.4085 J. Bellm (Lund U.), Science Coffee, 25.1.2018 Start simple Feynman gave a set of ‚simple‘ rules how to get cross sections from Lagrangians - extract rules from Lagrangian - draw diagrams and apply rules - average incoming possibilities - sum all final possibilities (once) J. Bellm (Lund U.), Science Coffee, 25.1.2018 Bit more complicated (fixed order) 100 50 0.2 0.4 0.6 0.8 1.0 -50 But: Divergences arise! UV —> Solved by renormalisation. -

Chapter 5. Characters: Typology and Page Encoding 1
Chapter 5. Characters: typology and page encoding 1 Chapter 5. Characters: typology and encoding Version 2.0 (16 May 2008) 5.1 Introduction PDF of chapter 5. The basic characters a-z / A-Z in the Latin alphabet can be encoded in virtually any electronic system and transferred from one system to another without loss of information. Any other characters may cause problems, even well established ones such as Modern Scandinavian ‘æ’, ‘ø’ and ‘å’. In v. 1 of The Menota handbook we therefore recommended that all characters outside a-z / A-Z should be encoded as entities, i.e. given an appropriate description and placed between the delimiters ‘&’ and ‘;’. In the last years, however, all major operating systems have implemented full Unicode support and a growing number of applications, including most web browsers, also support Unicode. We therefore believe that encoders should take full advantage of the Unicode Standard, as recommended in ch. 2.2.2 above. As of version 2.0, the character encoding recommended in The Menota handbook has been synchronised with the recommendations by the Medieval Unicode Font Initiative . The character recommendations by MUFI contain more than 1,300 characters in the Latin alphabet of potential use for the encoding of Medieval Nordic texts. As a consequence of the synchronisation, the list of entities which is part of the Menota scheme is identical to the one by MUFI. In other words, if a character is encoded with a code point or an entity in the MUFI character recommendation, it will be a valid character encoding also in a Menota text. -
Iq Is Ir Ip Io in Im Il It Ik Ii Ij Ih Ig If
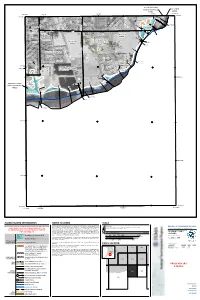
Sacramento County City Of Galt Unincorporated Areas 060264 060262 6 000m 6 000m 50 E 121°16'52.5" 121°18'45" 648000mE 49 E 38°15'00" 50 38°15'00" EET Y R 25 ST 99 4 A F 9 « 49 .8 W 6 T N 5 H O T CRYSTAL WAY S T E S R G H T D A S N O W T L D 4 E A T G ST E S 26 i 27 H D A T T 4 Dry Creek A C S 6 4 T S L . 8 T 9 3 S E (Near Galt) T R 2 M H SILVERADO WAY S N D 1 D C ZONE AE S R S T ALE AVE U GLEND T 4 H 7 C SPUR WAY H ST 47 NEW HOPE RD LIBERTY WAY SOUTHDALE CT City Of Galt i 060264 S iR 46 . 2 ) 4 6.2 ) Sacramento County ) ) ) City Of Galt ) CINDY LN Unincorporated Areas CORNELL RD ) Bridge 060264 ) 060262 Bridge L I N C B R O i E Q D DOWNING DR L R 4 Y 5 N M N .7 O A A G W J R A L A I Y Y R A L D CARDOSO DR W CARDOSO CT O N A Y R W D R R A E Y a T i V R l O r o D A GERMAINE DR a L D d A E i R 35 P I P A O T S 4 L L T 5 PESTANA DR P D TR T R . -

Special Characters in Aletheia
Special Characters in Aletheia Last Change: 28 May 2014 The following table comprises all special characters which are currently available through the virtual keyboard integrated in Aletheia. The virtual keyboard aids re-keying of historical documents containing characters that are mostly unsupported in other text editing tools (see Figure 1). Figure 1: Text input dialogue with virtual keyboard in Aletheia 1.2 Due to technical reasons, like font definition, Aletheia uses only precomposed characters. If required for other applications the mapping between a precomposed character and the corresponding decomposed character sequence is given in the table as well. When writing to files Aletheia encodes all characters in UTF-8 (variable-length multi-byte representation). Key: Glyph – the icon displayed in the virtual keyboard. Unicode – the actual Unicode character; can be copied and pasted into other applications. Please note that special characters might not be displayed properly if there is no corresponding font installed for the target application. Hex – the hexadecimal code point for the Unicode character Decimal – the decimal code point for the Unicode character Description – a short description of the special character Origin – where this character has been defined Base – the base character of the special character (if applicable), used for sorting Combining Character – combining character(s) to modify the base character (if applicable) Pre-composed Character Decomposed Character (used in Aletheia) (only for reference) Combining Glyph