Expression in Escherichia Coli of Cytochrome C Reductase Activity from a Maize NADH:Nitrate Reductase Complementary DNA'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -
![And Detoxification (Benzola]Pyrene Quinones/Oxygen Radicals/NADPH-Cytochrome P-450 Reductase) PAUL L](https://docslib.b-cdn.net/cover/7447/and-detoxification-benzola-pyrene-quinones-oxygen-radicals-nadph-cytochrome-p-450-reductase-paul-l-837447.webp)
And Detoxification (Benzola]Pyrene Quinones/Oxygen Radicals/NADPH-Cytochrome P-450 Reductase) PAUL L
Proc. Nati. Acad. Sci. USA Vol. 81, pp. 1696-1700, March 1984 Biochemistry Mutagenicity of quinones: Pathways of metabolic activation and detoxification (benzola]pyrene quinones/oxygen radicals/NADPH-cytochrome P-450 reductase) PAUL L. CHESIS*, DAVID E. LEVIN*, MARTYN T. SMITHt, LARS ERNSTERt, AND BRUCE N. AMES* Departments of *Biochemistry and tBiomedical and Environmental Health Sciences, School of Public Health, University of California, Berkeley, CA 94720; and tDepartment of Biochemistry, Arrhenius Laboratory, University of Stockholm, S-10691 Stockholm, Sweden Contributed by Bruce N. Ames, December 12, 1983 ABSTRACT The mutagenicity of various quinones, a class nones might also be mutagenic, and we have tested this pos- of compounds widely distributed in nature, is demonstrated in sibility using the TA104 strain, which is sensitive to a wide the Salmonella TA104 tester strain. The metabolic pathways variety of oxidative mutagens (17). We have also attempted by which four quinones, menadione, benzo[alpyrene 3,6-qui- to characterize the pathways by which several different qui- none, 9,10-phenanthrenequinone, and danthron, caused mu- nones are metabolized and to study the potential mutagenic- tagenicity in this test system were investigated in detail as were ity of the metabolites and side products formed. We there- the detoxification pathways. The two-electron reduction of fore decided to investigate only those quinones that required these quinones by NAD(P)H-quinone oxidoreductase (DT-di- metabolic activation to exhibit mutagenicity. To limit the aphorase) was not mutagenic, whereas the one-electron reduc- scope of this project we also chose not to study quinones tion, catalyzed by NADPH-cytochrome P-450 reductase, was that possess reactive leaving groups. -

Cytochrome C, Cytochrome B5 and Electron Transfer
Cytochrome c, cytochrome b5: electron transfer and new functions 1 Iron Protoporphyrin IX, heme b Heme a Prosthetic group Heme a is a form of heme found A flat and planar structure. In cytochromes a and a3. -. Itself Toxic O2 , H2O2 are formed. Insoluble 2 Covalent bonds Cytochrome c is a major player in membrane associated electron transport systems in bacteria and mitochondria. 3 Myoglobin, Hemoglobin His E7 Distal side Heme iron His F8 Proximal side Myoglobin has a strong affinity for oxygen Heme is located in the hydrophobic site. when it is in the lungs, and where the pressure is around 100 torr. When it reaches Heme Fe interacts with His. the tissues, where it's around 20 torr, the Coordination bond affinity for oxygen is still quite high. This makes myoglobin less efficient of an oxygen Propionates of heme interact with Arg. transporter than hemoglobin, which loses it's Ionic bond affinity for oxygen as the pressure goes down and releases the oxygen into the tissues. Heme is easily dissociated from the protein. Myoglobin's strong affinity for oxygen means that it keeps the oxygen binded to itself His (E7) is the oxygen binding site. instead of releasing it into the tissues. 4 Sickle cell anemia & malaria resistance In sickle cell hemoglobin (HbS) glutamic acid in position 6 (in beta chain) is mutated to valine. This change allows the deoxygenated form of the hemoglobin to stick to itself. 5 Figure 1. HO-1 Protects against Severe Malaria Using a mouse model for cerebral malaria, Ferreira et al. (2011) suggest a biochemical basis for the link between sickle cell disease and severe malaria. -

Supplementary Material
Supplementary material: Figure S1. Protein-Protein Interactions of the 127 Dysregulated proteins in brain tissues following IONP exposure. The graph was generated from STRING database. 125 nodes, 183 edges, 2.93 Average node degree, 0.411 Avg. local clustering coefficient, 122 Expected number of edges, PPI enrichment p-value 1.83e-07. 1 Figure S2. Protein-Protein Interactions of the 66 Dysregulated proteins in liver tissues following IONP exposure. The graph was generated from STRING database. 61 nodes, 103 edges, 3.38 Average node degree, 0.412 Avg. local clustering coefficient, 22 Expected number of edges, PPI enrichment p-value < 1.0e-16. 2 Figure S3. Protein-Protein Interactions of the 84 Dysregulated proteins in lung tissues following IONP exposure. The graph was generated from STRING database. 82 nodes, 125 edges, 3.05 Average node degree, 0.422 Avg. local clustering coefficient, 64 Expected number of edges, PPI enrichment p-value 1.07e-11. 3 Table S1. List of all dysregulated proteins in brain tissues following IONP exposure. A) Down regulated proteins and B) Upregulated proteins. Statistically significant iTRAQ ratios (p-value ratio and p-value sample≤0.05) for the 127 proteins that are dysregulated. A) Sequence Peptide Spectral P-Value P-Value Log 10 Accession Name Description Gene Coverage Ratio Count Count Ratio Sample Ratio % P00762 TRY1_RAT Anionic trypsin-1 Prss1 1 8 8.13 0.526 0.001 0.004 -0.279 P08592 A4_RAT Amyloid beta A4 protein App 1 2 1.17 0.546 0.010 0.004 -0.263 Q62696 DLG1_RAT Disks large homolog 1 Dlg1 1 1 1.32 -

Ferroptosis-Related Flavoproteins: Their Function and Stability
International Journal of Molecular Sciences Review Ferroptosis-Related Flavoproteins: Their Function and Stability R. Martin Vabulas Charité-Universitätsmedizin, Institute of Biochemistry, Charitéplatz 1, 10117 Berlin, Germany; [email protected]; Tel.: +49-30-4505-28176 Abstract: Ferroptosis has been described recently as an iron-dependent cell death driven by peroxida- tion of membrane lipids. It is involved in the pathogenesis of a number of diverse diseases. From the other side, the induction of ferroptosis can be used to kill tumor cells as a novel therapeutic approach. Because of the broad clinical relevance, a comprehensive understanding of the ferroptosis-controlling protein network is necessary. Noteworthy, several proteins from this network are flavoenzymes. This review is an attempt to present the ferroptosis-related flavoproteins in light of their involvement in anti-ferroptotic and pro-ferroptotic roles. When available, the data on the structural stability of mutants and cofactor-free apoenzymes are discussed. The stability of the flavoproteins could be an important component of the cellular death processes. Keywords: flavoproteins; riboflavin; ferroptosis; lipid peroxidation; protein quality control 1. Introduction Human flavoproteome encompasses slightly more than one hundred enzymes that par- ticipate in a number of key metabolic pathways. The chemical versatility of flavoproteins relies on the associated cofactors, flavin mononucleotide (FMN) and flavin adenine dinu- cleotide (FAD). In humans, flavin cofactors are biosynthesized from a precursor riboflavin that has to be supplied with food. To underline its nutritional essentiality, riboflavin is called vitamin B2. In compliance with manifold cellular demands, flavoproteins have been accommo- Citation: Vabulas, R.M. dated to operate at different subcellular locations [1]. -

Trans-Plasma Membrane Electron Transport System in the Myelin Membrane
Wilfrid Laurier University Scholars Commons @ Laurier Theses and Dissertations (Comprehensive) 2015 Characterization of the Trans-plasma Membrane Electron Transport System in the Myelin Membrane Afshan Sohail Wilfrid Laurier University, [email protected] Follow this and additional works at: https://scholars.wlu.ca/etd Part of the Biochemistry Commons, Molecular and Cellular Neuroscience Commons, and the Molecular Biology Commons Recommended Citation Sohail, Afshan, "Characterization of the Trans-plasma Membrane Electron Transport System in the Myelin Membrane" (2015). Theses and Dissertations (Comprehensive). 1694. https://scholars.wlu.ca/etd/1694 This Thesis is brought to you for free and open access by Scholars Commons @ Laurier. It has been accepted for inclusion in Theses and Dissertations (Comprehensive) by an authorized administrator of Scholars Commons @ Laurier. For more information, please contact [email protected]. Characterization of the Trans-plasma Membrane Electron Transport System in the Myelin Membrane By Afshan Sohail THESIS Submitted to the Department of Chemistry Faculty of Science In partial fulfillment of the requirements for Master of Science in Chemistry Wilfrid Laurier University 2014 © Afshan Sohail 2014 Abstract Myelination is the key feature of evolution in the nervous system of vertebrates. Myelin is the protrusion of glial cells. More specifically, "oligodendrocytes" in the central nervous system (CNS), and "Schwann" cells in the peripheral nervous system (PNS) form myelin membranes. Myelin remarkably, enhances the propagation of nerve impulses. However, myelin restricts the access of extracellular metabolites to the axons. A pathology called "demyelination" is associated with myelin. The myelin sheath is not only an insulator, but it is itself metabolically active. In this study it is hypothesized that the ratio of NAD(P)+/NAD(P)H and the glycolytic pathway of the myelin sheath is maintained via trans-plasma membrane electron transport system (t-PMET). -

Genomic Evidence of Reactive Oxygen Species Elevation in Papillary Thyroid Carcinoma with Hashimoto Thyroiditis
Endocrine Journal 2015, 62 (10), 857-877 Original Genomic evidence of reactive oxygen species elevation in papillary thyroid carcinoma with Hashimoto thyroiditis Jin Wook Yi1), 2), Ji Yeon Park1), Ji-Youn Sung1), 3), Sang Hyuk Kwak1), 4), Jihan Yu1), 5), Ji Hyun Chang1), 6), Jo-Heon Kim1), 7), Sang Yun Ha1), 8), Eun Kyung Paik1), 9), Woo Seung Lee1), Su-Jin Kim2), Kyu Eun Lee2)* and Ju Han Kim1)* 1) Division of Biomedical Informatics, Seoul National University College of Medicine, Seoul, Korea 2) Department of Surgery, Seoul National University Hospital and College of Medicine, Seoul, Korea 3) Department of Pathology, Kyung Hee University Hospital, Kyung Hee University School of Medicine, Seoul, Korea 4) Kwak Clinic, Okcheon-gun, Chungbuk, Korea 5) Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, Uijeongbu, Korea 6) Department of Radiation Oncology, Seoul St. Mary’s Hospital, Seoul, Korea 7) Department of Pathology, Chonnam National University Hospital, Kwang-Ju, Korea 8) Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea 9) Department of Radiation Oncology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea Abstract. Elevated levels of reactive oxygen species (ROS) have been proposed as a risk factor for the development of papillary thyroid carcinoma (PTC) in patients with Hashimoto thyroiditis (HT). However, it has yet to be proven that the total levels of ROS are sufficiently increased to contribute to carcinogenesis. We hypothesized that if the ROS levels were increased in HT, ROS-related genes would also be differently expressed in PTC with HT. To find differentially expressed genes (DEGs) we analyzed data from the Cancer Genomic Atlas, gene expression data from RNA sequencing: 33 from normal thyroid tissue, 232 from PTC without HT, and 60 from PTC with HT. -

Structural Basis of Inter-Domain Electron Transfer in Ncb5or, a Redox Enzyme Implicated in Diabetes and Lipid Metabolism
STRUCTURAL BASIS OF INTER-DOMAIN ELECTRON TRANSFER IN NCB5OR, A REDOX ENZYME IMPLICATED IN DIABETES AND LIPID METABOLISM By Bin Deng Submitted to the graduate degree program in Rehabilitation Science and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy Hao Zhu, Ph.D. (co-chair, advisor) Irina Smirnova, Ph.D. (co-chair) David Benson, Ph.D. (co-advisor) WenFang Wang, Ph.D. Aron Fenton, Ph.D. Date defended: August 17, 2011 The Dissertation Committee for Bin Deng certifies that this is the approved version of the following dissertation STRUCTURAL BASIS OF INTER-DOMAIN ELECTRON TRANSFER IN NCB5OR, A REDOX ENZYME IMPLICATED IN DIABETES AND LIPID METABOLISM Hao Zhu, Ph.D. (co-chair, advisor) Irina Smirnova, Ph.D. (co-chair) Date approved: August 22, 2011 ii ABSTRACT NADH cytochrome b5 oxidoreductase (Ncb5or) is a multi-domain redox enzyme found in all animal tissues and associated with the endoplasmic reticulum (ER). Ncb5or contains (from N-terminus to C terminus) a novel N-terminal region, the b5 domain (Ncb5or-b5), the CS domain, and the b5R domain (Ncb5or-b5R). Ncb5or-b5, the heme binding domain, is homologous to microsomal cytochrome b5 (Cyb5A) and belongs to cytochrome b5 superfamily. Ncb5or-b5R, the FAD (flavin adenine dinucleotide) binding domain, is homologous to cytochrome b5 reductase (Cyb5R3) and belongs to ferredoxin NADP+ reductase superfamily. Both superfamilies are of great biological significance whose members have important functions. The CS domain can be assigned into the heat shock protein 20 (HSP20, or p23) family, whose members are known to mediate protein-protein interactions. -

Adaptation of Cytochrome-B5 Reductase Activity and Methaemoglobinaemia in Areas with a High Nitrate Concentration in Drinking-Water S.K
Adaptation of cytochrome-b5 reductase activity and methaemoglobinaemia in areas with a high nitrate concentration in drinking-water S.K. Gupta,1 R.C. Gupta,2 A.K. Seth,3 A.B. Gupta,4 J.K. Bassin,5 & A. Gupta6 An epidemiological investigation was undertaken in India to assess the prevalence of methaemoglobinaemia in areas with high nitrate concentration in drinking-water and the possible association with an adaptation of cytochrome-b5 reductase. Five areas were selected, with average nitrate ion concentrations in drinking-water of 26, 45, 95, 222 and 459 mg/l. These areas were visited and house schedules were prepared in accordance with a statistically designed protocol. A sample of 10% of the total population was selected in each of the areas, matched for age and weight, giving a total of 178 persons in five age groups. For each subject, a detailed history was documented, a medical examination was conducted and blood samples were taken to determine methaemoglobin level and cytochrome-b5 reductase activity. Collected data were subjected to statistical analysis to test for a possible relationship between nitrate concentration, cytochrome-b5 reductase activity and methaemoglobinaemia. High nitrate concentrations caused methaemoglobinaemia in infants and adults. The reserve of cytochrome-b5 reductase activity (i.e. the enzyme activity not currently being used, but which is available when needed; for example, under conditions of increased nitrate ingestion) and its adaptation with increasing water nitrate concentration to reduce methaemoglobin were more pronounced in children and adolescents. Voir page 752 le reÂsume en francËais. En la pa gina 753 figura un resumen en espanÄ ol. -

View / Download
Samhan-Arias AK, López-Sánchez C, Marques-da-Silva D, Lagoa R, Garcia-Lopez V, García-Martínez Neuromedicine V, Gutierrez-Merino C, J Neurol Neuromedicine (2016) 1(6): 61-65 www.jneurology.com www.jneurology.com Journal of Neurology & Neuromedicine Mini Review Open Access Biochemical and anatomical basis of brain dysfunctions caused by cytochrome b5 reductase deficiency or dysregulation Alejandro K. Samhan-Arias1*, Carmen López-Sánchez2*, Dorinda Marques-da-Silva1, Ricardo Lagoa1-3, Virginio Garcia-Lopez2,4, Virginio García-Martínez2, Carlos Gutierrez-Merino1# 1Dept. Biochemistry and Molecular Biology, Faculty of Sciences, University of Extremadura, 06006-Badajoz, Spain 2Dept. Human Anatomy and Embryology, Faculty of Medicine, University of Extremadura, 06006-Badajoz, Spain 3ESTG- Polytechnic Institute of Leiria, Leiria, Portugal 4FARMADIEX 06008 Badajoz, Spain Article Info Article Notes ABSTRACT Received: August 29, 2016 Accepted: September 21, 2016 Cytochrome b5 reductase (Cb5R) and cytochrome b5 (Cb5) are coupled redox Keywords: systems with a high potential as biomarkers of health and disease in the brain *Correspondence: Delta9-Tetrahydrocannabinol Dr. Carlos Gutierrez-Merino because they regulate metabolic pathways that are essential to maintain normal Dronabinol Dept. Biochemistry and Molecular Biology, Faculty of Sciences, neuronal function, like lipid biosynthesis, steroid and xenobiotics metabolism, THC University of Extremadura, 06006-Badajoz, Spain, Phone/Fax: neuronal bioenergetics and production of reactive oxygen species. Mutations of Cannabidiol +34924289419. the Cb R reported in humans produce recessive congenital methemoglobinemia CBD 5 E-mail: [email protected] of type II, a disease with severe clinical neurological dysfunctions. The isoform 3 *Both authors have contributed equally to this work and should be of Cb5R (Cb5R3) and Cb5 are highly expressed in pyramidal neurons of the primary considered first authors and secondary motor areas of frontoparietal cerebral cortex, hippocampus, © 2016 Samhan-Arias AK and López-Sánchez C. -
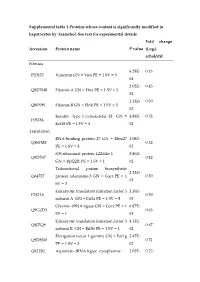
Supplemental Table 1 Proteins Whose Content Is Significantly Modified in Hepatocytes by Aramchol
Supplemental table 1 Proteins whose content is significantly modified in hepatocytes by Aramchol. See text for experimental details Fold change Accession Protein name P value (Log2) achol/ctrl Fibrosis 6.29E- 0.13 P20152 Vimentin GN = Vim PE = 1 SV = 3 04 2.02E- 0.45 Q8BTM8 Filamin-A GN = Flna PE = 1 SV = 5 02 1.13E- 0.50 Q80X90 Filamin-B GN = Flnb PE = 1 SV = 3 02 Keratin type I cytoskeletal 18 GN = 4.89E- 0.78 P05784 Krt18 PE = 1 SV = 5 02 Translation RNA-binding protein 27 GN = Rbm27 1.08E- Q5SFM8 0.32 PE = 1 SV = 3 02 60S ribosomal protein L22-like 1 3.86E- Q9D7S7 0.42 GN = Rpl22l1 PE = 1 SV = 1 02 Trifunctional purine biosynthetic 2.33E- Q64737 protein adenosine-3 GN = Gart PE = 1 0.50 03 SV = 3 Eukaryotic translation initiation factor 3 3.10E- P23116 0.59 subunit A GN = Eif3a PE = 1 SV = 5 03 Glycine--tRNA ligase GN = Gars PE = 1 6.87E- Q9CZD3 0.63 SV = 1 03 Eukaryotic translation initiation factor 3 4.13E- Q8JZQ9 0.67 subunit B GN = Eif3b PE = 1 SV = 1 02 Elongation factor 1-gamma GN = Eef1g 2.47E- Q9D8N0 0.71 PE = 1 SV = 3 02 Q922B2 Aspartate--tRNA ligase cytoplasmic 2.02E- 0.72 GN = Dars PE = 1 SV = 2 02 Arginine--tRNA ligase cytoplasmic 1.47E- Q9D0I9 0.72 GN = Rars PE = 1 SV = 2 02 RNA-binding protein 25 GN = Rbm25 1.28E- B2RY56 1.92 PE = 1 SV = 2 02 Eukaryotic translation initiation factor 4 7.92E- Q80XI3 3.90 gamma 3 GN = Eif4g3 PE = 1 SV = 2 03 Lipid metabolism Prostaglandin reductase 1 GN = Ptgr1 4.55E- Q91YR9 0.42 PE = 1 SV = 2 02 3.09E- Q9DBG5 Perilipin-3 GN = Plin3 PE = 1 SV = 1 0.51 02 Acyl-CoA dehydrogenase family 3.98E- -

Interactions Between Redox Partners in Various Cytochrome P450 Systems: Functional and Structural Aspects
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1460 (2000) 353^374 www.elsevier.com/locate/bba Interactions between redox partners in various cytochrome P450 systems: functional and structural aspects David F.V. Lewis a;*, Peter Hlavica b a Molecular Toxicology Group, School of Biological Sciences, University of Surrey, Guildford, Surrey GU2 5XH, UK b Walther Straub-Institute fu«r Pharmakologie and Toxikologie, Universita«tMu«nchen, Nussbaumstrasse 26, D-8033 Mu«nchen, Germany Received 1 December 1999; received in revised form 28 July 2000; accepted 4 August 2000 Abstract The various types of redox partner interactions employed in cytochrome P450 systems are described. The similarities and differences between the redox components in the major categories of P450 systems present in bacteria, mitochondria and microsomes are discussed in the light of the accumulated evidence from X-ray crystallographic and NMR spectroscopic determinations. Molecular modeling of the interactions between the redox components in various P450 mono-oxygenase systems is proposed on the basis of structural and mutagenesis information, together with experimental findings based on chemical modification of key residues likely to be associated with complementary binding sites on certain typical P450 isoforms and their respective redox partners. ß 2000 Elsevier Science B.V. All rights reserved. Keywords: Cytochrome P450; Redox partner interaction; Electron transfer rate 1. Introduction istics in keeping with their functionality as mono- oxygenases, it is apparent that di¡erences exist in Enzymes of the cytochrome P450 (CYP) superfam- their electron transport chains when one compares ily of heme-thiolate proteins are found in most bio- CYP systems from eukaryotic and prokaryotic or- logical species from all ¢ve kingdoms (monera, pro- ganisms [3,4].