Huhtamaki Report 2018 V1 93.Docx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This First Determination of Terrestrial Heat Flow in Norwegian Lakes Was Car Ried out by the Niedersachsische Landesamt Flir B
Terrestrial Heat Flow Determinations from Lakes in Southern Norway* RALPH HÅNEL, GISLE GRØNLI E & KNUTS. HEI ER Hiinel, R., Grønlie, G. & Heier, K. S.: Terrestrial heat flow determinations from !akes in southern Norway. Norsk Geologisk Tidsskrift, Vol. 54, pp. 423-428. Oslo 1974. Twenty-four heat flow determinations based on measurements in !akes are presented from southern Norway. All the measurements Iie within the Pre cambrian Baltic Shield and the Permian Oslo Graben. The mean value, 0.96 ± 0.21 hfu (l hfu = 1(}6 cal/cm2s), is in good agreement with previously published results from both Norway and the Baltic Shield in general. The results give additional evidence in favour of the suggested presence of a zone of anomalous low mantle heat flow to the east of the Caledonian mountains in Norway. R. Hiinel, Niedersiichsisches Landesamt fiir Bodenforschung, 3 Hannover 23, West Germany. G. GrØnlie, Institutt for geologi, Universitetet i Oslo, Blindern, Oslo 3, Norway. K.S. Heier, Mineralogisk-geologisk museum, Sars gt. l, Oslo 5, Norway. This first determination of terrestrial heat flow in Norwegian lakes was car ried out by the Niedersachsische Landesamt flir Bodenforschung in Han nover, West Germany in cooperation with Institutt for geologi and Minera logisk-geologisk museum at Universitetet i Oslo, Norway. When the measurements began, a project of determining heat flow from boreholes on land had been going for some time, and the first results from this study have now been published (Swanberg et al. 1974). Swanberg et al. present 15 heat flow values of which 11 are from southern Norway and are relevant to this study. -

Avd. II Regionale Og Lokale Forskrifter Mv
Nr. 1 - 2003 Side 1 - 206 NORSK LOVTIDEND Avd. II Regionale og lokale forskrifter mv. Nr. 1 Utgitt 14. april 2003 Innhold Side Forskrifter 2000 Feb. 1. Forskrift om snøscooterløyper, Nordreisa (Nr. 1708) ............................................................ 1 Nov. 9. Forskrift om snøscooterløyper, Bardu (Nr. 1709)................................................................... 2 2001 Juni 13. Forskrift om vedtekter for Masfjorden kraftfond, Masfjorden (Nr. 1700) ............................. 3 2002 Des. 17. Forskrift om gebyrregulativ for teknisk sektor, Masfjorden (Nr. 1830)................................. 4 Des. 18. Forskrift om kommunale barnehager, Oslo (Nr. 1831) ........................................................... 4 Des. 19. Forskrift om utvidet jakttid på kanadagås og stripegås, Farsund (Nr. 1832).......................... 8 Des. 19. Forskrift om åpen brenning og brenning av avfall i småovner, Rissa (Nr. 1833).................... 8 Des. 20. Forskrift om vann- og avløpsgebyrer, Modum (Nr. 1834)...................................................... 9 Des. 23. Forskrift om tillatte vekter og dimensjoner for kjøretøy på fylkes- og kommunale veger (vegliste for Nord-Trøndelag), Nord-Trøndelag (Nr. 1835) .................................................. 9 Sep. 11. Forskrift for adressetildeling, Bjerkreim (Nr. 1849) .............................................................. 9 Okt. 29. Forskrift om åpen brenning og brenning av avfall i småovner, Drammen (Nr. 1850) ........... 10 Nov. 6. Forskrift om utvidet jakttid -

Miljøgifter I Store Norske Innsjøer, 2015 Environmental Pollutants in Large Norwegian Lakes, 2015
MILJØOVERVÅKNING M-548 | 2016 Miljøgifter i store norske innsjøer, 2015 Forekomst og biomagnifisering i fisk og zooplankton KOLOFON Utførende institusjon Norsk institutt for vannforskning (NIVA), Norsk institutt for luftforskning (NILU) Oppdragstakers prosjektansvarlig Kontaktperson i Miljødirektoratet Eirik Fjeld Eivind Farmen M-nummer År Sidetall Miljødirektoratets kontraktnummer M-548 2016 97 15078019, 16078018 Utgiver Prosjektet er finansiert av NIVA; Rapport 7062-2016; Prosjekt O-13223; Miljødirektoratet ISBN 978-577-6797-6 Forfatter(e) Fjeld, Eirik (NIVA), Bæk, Kine (NIVA), Rognerud, Sigurd (NIVA), Rundberget, Jan Thomas (NIVA), Schlabach, Martin (NILU) og Warner, Nicholas A. (NILU) Tittel – norsk og engelsk Miljøgifter i store norske innsjøer, 2015 Environmental pollutants in large Norwegian lakes, 2015 Sammendrag – summary Vi rapporterer her om forekomsten av miljøgifter i de pelagiske næringskjedene i innsjøene Mjøsa, Randsfjorden og Femunden, samt i supplerende materiale av fisk fra Tyrifjorden og Vansjø, innsamlet i 2015. Kvikksølv og organiske miljøgifter (cVMS, PCB, PBDE, PFAS) ble analysert i prøver av fisk fra alle sjøene, samt i planktoniske krepsdyr i Mjøsa. Av hovedfunnene nevnes at siloksanforbindelsene D5 og D6 viser trofisk magnifisering i Mjøsa og Randsfjorden, samt at PFTrA er den dominerende PFAS-forbindelsen i leverprøver av fisk. We report here on the presence of contaminants in the pelagic food chains in the lakes Mjøsa, Randsfjorden and Femunden and in supplementary material of fish from Tyrifjorden and Vansjø, sampled in 2015. Mercury and persistent organic pollutants (cVMS, PCBs, PBDEs, PFAS) were analyzed in samples of fish from all lakes, as well as pelagic crustaceans in Mjøsa. Among the key findings are that the siloxanes D5 and D6 show trophic magnification in Mjøsa and Randsfjorden and that PFTrA is the dominating PFAS in liver samples of fish. -

Rapport Fra Vitenskapelig Råd for Lakseforvaltning Nr 8
RAPPORT FRA VITENSKAPELIG RÅD FOR LAKSEFORVALTNING NR 8 Status for norske laksebestander i 2015 RAPPORT FRA VITENSKAPELIG RÅD FOR LAKSEFORVALTNING NR. 8 RAPPORT FRA VITENSKAPELIG RÅD FOR LAKSEFORVALTNING NR 8 Status for norske laksebestander i 2015 1 RAPPORT FRA VITENSKAPELIG RÅD FOR LAKSEFORVALTNING NR. 8 RAPPORTEN REFERERES SOM Anon. 2015. Status for norske laksebestander i 2015. Rapport fra Vitenskapelig råd for lakseforvaltning nr 8, 300 s. Trondheim, juni 2015 ISSN: 1891-442X ISBN: 978-82-93038-15-3 RETTIGHETSHAVER © Vitenskapelig råd for lakseforvaltning www.vitenskapsradet.no REDAKSJON Eva B. Thorstad & Torbjørn Forseth TILGJENGELIGHET Åpen PUBLISERINGSTYPE Digitalt dokument (pdf) NØKKELORD Laks - Salmo salar - sjøørret - Salmo trutta - beskatning - gytebestandsmål - forvaltningsmål - bestandsutvikling - bestandsstatus - beskatningsrater - fangststatistikk - høsting - høstbart overskudd - innsigsestimat - PFA - marin overlevelse - trusselfaktorer - rømt oppdrettslaks - Gyrodactylus salaris 2 RAPPORT FRA VITENSKAPELIG RÅD FOR LAKSEFORVALTNING NR. 8 INNHOLD HOVEDFUNN ............................................................................................................... 7 SAMMENDRAG AV RAPPORTEN .............................................................................. 8 VITENSKAPELIG RÅD FOR LAKSEFORVALTNING ............................................. 18 MEDLEMMER AV VITENSKAPELIG RÅD FOR LAKSEFORVALTNING ........... 19 1 INNLEDNING ....................................................................................................... -

Spredning Av Ferskvannsfisk I Norge 1205 En Fylkesvis Oversikt Og Nye Registreringer I 2015
Spredning av ferskvannsfisk i Norge 1205 En fylkesvis oversikt og nye registreringer i 2015 Trygve Hesthagen og Odd Terje Sandlund NINAs publikasjoner NINA Rapport Dette er en elektronisk serie fra 2005 som erstatter de tidligere seriene NINA Fagrapport, NINA Oppdragsmelding og NINA Project Report. Normalt er dette NINAs rapportering til oppdragsgiver etter gjennomført forsknings-, overvåkings- eller utredningsarbeid. I tillegg vil serien favne mye av instituttets øvrige rapportering, for eksempel fra seminarer og konferanser, resultater av eget forsk- nings- og utredningsarbeid og litteraturstudier. NINA Rapport kan også utgis på annet språk når det er hensiktsmessig. NINA Temahefte Som navnet angir behandler temaheftene spesielle emner. Heftene utarbeides etter behov og se- rien favner svært vidt; fra systematiske bestemmelsesnøkler til informasjon om viktige problemstil- linger i samfunnet. NINA Temahefte gis vanligvis en populærvitenskapelig form med mer vekt på illustrasjoner enn NINA Rapport. NINA Fakta Faktaarkene har som mål å gjøre NINAs forskningsresultater raskt og enkelt tilgjengelig for et større publikum. De sendes til presse, ideelle organisasjoner, naturforvaltningen på ulike nivå, politikere og andre spesielt interesserte. Faktaarkene gir en kort framstilling av noen av våre viktigste forsk- ningstema. Annen publisering I tillegg til rapporteringen i NINAs egne serier publiserer instituttets ansatte en stor del av sine viten- skapelige resultater i internasjonale journaler, populærfaglige bøker og tidsskrifter. Spredning av ferskvannsfisk i Norge En fylkesvis oversikt og nye registreringer i 2015 Trygve Hesthagen og Odd Terje Sandlund Norsk institutt for naturforskning NINA Rapport 1205 Hesthagen, T. & Sandlund, O.T. 2016. Spredning av ferskvanns- fisk i Norge. En fylkesvis oversikt og nye registreringer i 2015. NINA Rapport 1205. -

Mapping the Occurrence of Gyrodactylus Salaris Upstream of the Natural Anadromous Region of the Drammenselva Catchment 2018 NORWEGIAN VETERINARY INSTITUTE
Annual Report Mapping the occurrence of Gyrodactylus salaris upstream of the natural anadromous region of the Drammenselva catchment 2018 NORWEGIAN VETERINARY INSTITUTE Mapping the occurrence of Gyrodactylus salaris upstream of the natural anadromous region of the Drammenselva catchment, 2018 Contents Summary ...................................................................................................................... 3 Introduction .................................................................................................................. 3 Aims ........................................................................................................................... 4 Materials and methods ..................................................................................................... 4 Results and discussion ...................................................................................................... 5 References ................................................................................................................... 6 Authors Commissioned by Sigurd Hytterød, Mari Darrud, Saima Nasrin Norwegian Food Safety Authority Mohammed, Johannes Rusch and Haakon Hansen ISSN 1894-5678 Design Cover: Reine Linjer © Norwegian Veterinary Institute 2019 Photo front page: Johannes Rusch/Haakon Hansen Surveillance programmes in Norway – Gyrodactylus salaris-Drammenselva catchment – Annual Report 2018 2 NORWEGIAN VETERINARY INSTITUTE Summary This report presents results from a surveillance programme that aims to map -
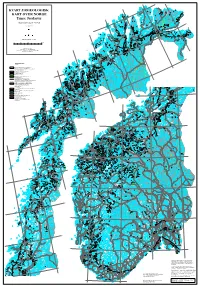
Jordarter V E U N O T N a Leirpollen
30°E 71°N 28°E Austhavet Berlevåg Bearalváhki 26°E Mehamn Nordkinnhalvøya KVARTÆRGEOLOGISK Båtsfjord Vardø D T a e n a Kjøllefjord a n f u j o v r u d o e Oksevatnet t n n KART OVER NORGE a Store L a Buevatnet k Geatnjajávri L s Varangerhalvøya á e Várnjárga f g j e o 24°E Honningsvåg r s d Tema: Jordarter v e u n o t n a Leirpollen Deanodat Vestertana Quaternary map of Norway Havøysund 70°N en rd 3. opplag 2013 fjo r D e a T g tn e n o a ra u a a v n V at t j j a n r á u V Porsanger- Vadsø Vestre Kjæsvatnet Jakobselv halvøya o n Keaisajávri Geassájávri o Store 71°N u Bordejávrrit v Måsvatn n n i e g Havvannet d n r evsbotn R a o j s f r r Kjø- o Bugøy- e fjorden g P fjorden 22°E n a Garsjøen Suolo- s r Kirkenes jávri o Mohkkejávri P Sandøy- Hammerfest Hesseng fjorden Rypefjord t Bjørnevatn e d n Målestokk (Scale) 1:1 mill. u Repparfjorden s y ø r ø S 0 25 50 100 Km Sørøya Sør-Varanger Sállan Skáiddejávri Store Porsanger Sametti Hasvik Leaktojávri Kartet inngår også i B áhèeveai- NASJONALATLAS FOR NORGE 20°E Leavdnja johka u a Lopphavet -

Overvåking Av Fiskebestander I Store Innsjøer 1274 Metodeutprøving Og Anbefalinger
Overvåking av fiskebestander i store innsjøer 1274 Metodeutprøving og anbefalinger Odd Terje Sandlund (red.), Åge Brabrand, Karl Øystein Gjelland, Lars Erik Høitomt, Arne N. Linløkken, Kjetil Olstad, Oskar Pettersen, Atle Rustadbakken NINAs publikasjoner NINA Rapport Dette er en elektronisk serie fra 2005 som erstatter de tidligere seriene NINA Fagrapport, NINA Oppdragsmelding og NINA Project Report. Normalt er dette NINAs rapportering til oppdragsgiver etter gjennomført forsknings-, overvåkings- eller utredningsarbeid. I tillegg vil serien favne mye av instituttets øvrige rapportering, for eksempel fra seminarer og konferanser, resultater av eget forsk- nings- og utredningsarbeid og litteraturstudier. NINA Rapport kan også utgis på annet språk når det er hensiktsmessig. NINA Temahefte Som navnet angir behandler temaheftene spesielle emner. Heftene utarbeides etter behov og se- rien favner svært vidt; fra systematiske bestemmelsesnøkler til informasjon om viktige problemstil- linger i samfunnet. NINA Temahefte gis vanligvis en populærvitenskapelig form med mer vekt på illustrasjoner enn NINA Rapport. NINA Fakta Faktaarkene har som mål å gjøre NINAs forskningsresultater raskt og enkelt tilgjengelig for et større publikum. De sendes til presse, ideelle organisasjoner, naturforvaltningen på ulike nivå, politikere og andre spesielt interesserte. Faktaarkene gir en kort framstilling av noen av våre viktigste forsk- ningstema. Annen publisering I tillegg til rapporteringen i NINAs egne serier publiserer instituttets ansatte en stor del av sine viten- skapelige resultater i internasjonale journaler, populærfaglige bøker og tidsskrifter. Overvåking av fiskebestander i store innsjøer Metodeutprøving og anbefalinger Odd Terje Sandlund (red.), Åge Brabrand, Karl Øystein Gjelland, Lars Erik Høitomt, Arne N. Linløkken, Kjetil Olstad, Oskar Pettersen, Atle Rustadbakken Norsk institutt for naturforskning NINA Rapport xxx Sandlund, O.T. -

Forslag Til Verneplan for Tyrifjorden
KONGELIG RESOLUSJON Klima- og miljødepartementet Ref.nr.: Statsråd: Ola Elvestuen Saksnr.: Dato: 22.06.2018 Forslag til verneplan for Tyrifjorden 1 FORSLAG Klima- og miljødepartementet legger med dette fram forslag til verneplan for Tyrifjorden. Verneforslaget omfatter 7 naturreservat og 6 biotopvernområder. Forslaget omfatter ca. 16,3 km2 nytt vern. 5,1 km2 er vernet pr. i dag slik at verneplan for Tyrifjorden til sammen vil omfatte ca. 21,4 km². Av dette er ca. 20,1 km2 ferskvann. Verneforslaget omfatter: - Storøysundet – Sælabonn naturreservat ca. 5733 dekar, Hole kommune - Steinfjorden biotopvernområde ca. 3825 dekar, Hole og Ringerike kommuner - Steinsvika naturreservat ca. 263 dekar, Hole kommune - Søndre Tyrifjorden naturreservat ca. 5848 dekar, Modum og Ringerike kommuner - Vikersund – Bergsjø biotopvernområde ca. 3232 dekar, Modum kommune - Vassbunn naturreservat ca. 119 dekar, Modum kommune - Ådalselva naturreservat ca. 2012 dekar, Ringerike kommune - Hovsenga naturreservat ca. 337 dekar, Ringerike kommune - Solbergtjern naturreservat ca. 48 dekar, Ringerike kommune - Lienskjæret biotopvernområde ca. 8 dekar, Hole kommune - Furuøyene biotopvernområde ca. 15 dekar, Ringerike kommune - Væleren biotopvernområde ca. 106 dekar, Ringerike kommune - Storøya biotopvernområde ca. 29 dekar, Lier kommune 1.1 Hjemmelsgrunnlag Områdene foreslås vernet som henholdsvis naturreservat og biotopvernområde i medhold av naturmangfoldloven §§, 34, 37, 38 og 62. Vilkåret for å kunne opprette et naturreservat etter naturmangfoldloven § 37 er at arealet enten inneholder truet, sjelden eller sårbar natur, representerer en bestemt naturtype, på annen måte har særlig betydning for biologisk mangfold, utgjør en spesiell geologisk forekomst eller har særskilt naturvitenskaplig verdi. De foreslåtte naturreservatene er alle våtmarksområder som har funksjon enten som hekke-, nærings-, overvintrings-, raste- eller trekkområde eller som en kombinasjon av flere av disse funksjonene. -

Dolmen D. & Kleiven E. 1997
ZOOLOGISK NOTAT 1997-2 EL VEMUSLINGEN MARGARITIFERA MARGARITIFERA I NORGE 2 Dag Dolmen Einar Kleiven NORGES TEKNISK-NATURVITENSKAPELIGE UNIVERSITET VITENSKAPSMUSEET TRONDHEIM Zoolo gisk notat 1997-2 Elvemuslingen Margaritifera n1.argaritifera i Norge 2 Dag Dolmen Einar Kleiven Norges teknisk-naturvitenskapelige universitet Vitenskapsmuseet Laboratoriet for ferskvannsøkologi og innlandsfiske (LFI, notat nr. 15) Trondheim, april 1997 ISBN 82-7126-529-6 ISSN 0803-0146 INNHOLD FORORD ........................................................................................................................... 5 l ~EDNING ........................................................................................................... 6 2 METODER OG MATERIALE.; ............. .................................................................... 7 3 RESULTATER .......................................................................................................... 8 ) ) 5 FORORD En av de mest interessante, men også sårbare, dyr i rennende vann her tillands er elvemus Hngen. Elvemuslingen har vernestatus som sårbar art, og det meldes om reproduksjons problemer og tilbakegang både på det europeiske kontinentet og i Skandinavia. Direktoratet for naturforvaltning (DN) har derfor delfinansiert en kartlegging av e1vemuslingen i Norge, bl.a. med tanke på en forvaltningsplan for arten. Denne delrapport 2 inneholder resultatene fra kartleggingsprosjektet, og er dessuten tenkt som et av grunnlagsdokumentene for en slik for valtningsplan. Kontaktpersoner ved DN -

Deltagere I Vannregionutvalget (VRU) I Innlandet Og Viken Vannregion Og
Deltagere i vannregionutvalget (VRU) i Innlandet og Viken vannregion og de norske delene av vannregion Västerhavet, inkludert varamedlemmer, som stiller på møtet 25.11.2020 Virksomhet Navn Funksjon/ Stilling Viken fylkeskommune / Anne Beathe Tvinnereim (Sp) Leder i vannregionutvalget / Vannregionmyndighet (VRM) fylkesråd plan, klima og miljø Viken fylkeskommune / Hilde Reine fylkesdirektør plan, klima og miljø Vannregionmyndighet (VRM) Viken fylkeskommune / Elin Tangen Skeide Leder i miljøavdelingen Vannregionmyndighet (VRM) Viken fylkeskommune / Helene Gabestad Rådgiver Vannregionmyndighet (VRM) Viken fylkeskommune / Tonje Rundbråten Rådgiver Vannregionmyndighet (VRM) Viken fylkeskommune / Estrella Fernandez Rådgiver Vannregionmyndighet (VRM) Viken fylkeskommune Hans Støvern / Jonas Rådgivere samferdsel Ramsrud Innlandet fylkeskommune Inger Torun Klosbøle (Ap) Deltager i politisk utvalg Innlandet fylkeskommune Mari Olsen Rådgiver miljø Innlandet fylkeskommune Claire Elizabeth Blant Rådgivere samferdsel Oslo kommune Venter på tilbakemelding Deltager i politisk utvalg Oslo kommune Gro Koppen Rådgiver i byrådsavdelingen Vestfold og Telemark Karoline Aarvold (H) Deltager i politisk utvalg fylkeskommune Vestfold og Telemark Lars W. Solheim Rådgiver fylkeskommune Cathrine Nedberg Rådgiver Trøndelag fylkeskommune Steinar Aspli (Sp) Deltager i politisk utvalg Trøndelag fylkeskommune Bjørn Borge Skei Rådgiver FM Oslo og Viken Hilde Sundt Skålevåg Seksjonssjef miljø FM Oslo og Viken Håvard Hornnæs (vara) Rådgiver miljø FM Oslo og Viken Otto Galleberg -

Nasjonalt Referansesystem for Landskap Beskrivelse Av Norges 45 Landskapsregioner
Nasjonalt referansesystem for landskap Beskrivelse av Norges 45 landskapsregioner av Oskar Puschmann, NIJOS Side 38 - 41 ”Landskapsregion 08 Innsjø- og silurbygdene på Østlandet” Referanse: Puschmann, Oskar. 2005. ”Nasjonalt referansesystem for landskap. Beskrivelse av Norges 45 landskapsregioner.” NIJOS- rapport 10/2005. Norsk institutt for jord- og skogkartlegging, Ås. Side 38-41. Nasjonalt referansesystem for landskap Beskrivelse av Norges 45 landskapsregioner Landskapsregion 08 Innsjø- og silurbygdene på Østlandet. NIJOS-rapport 10/2005 Side 38 Nasjonalt referansesystem for landskap Beskrivelse av Norges 45 landskapsregioner BE- LANDSKAPSREGION 08 INNSJØ- OG SILURBYGDENE PÅ TYD- NING ØSTLANDET Regionen består av seks underregioner 1 *** LANDSKAPETS Regionen mangler en enhetlig hovedform. Ulike lave dalformer er trolig mest karakter- ** HOVEDFORM istisk. Nordre del av Mjøsa, og det meste av Randsfjorden og Holsfjorden er eks. på * ikke enhetlig form trauformer eller u-daler med stort vannspeil i bunnen. Også Sokna har dalpreg, men her * ulike dal- og trau- er det et svakere relieff og dalsidene er mer sammensatt av større åser. Ringerike kan former, vannspeil betraktes som en gjennomgangsdal med sterkt asymmetrisk profil og vid dalbunn. Øst- og i noen grad sørsida av Tyrifjorden er delvis steil, mens vestsiden er slakt stigende. * bølgende sletter En asymmetrisk dal er også strekningen Gran - Lunner, og i noen grad også sørlige del av Randsfjorden. Områdene langs midtre del av Mjøsa har også en svak dalform, og det samme gjelder for områdene ved Eina. Regionen har også større områder på Hed- marken og Toten med hvelvete og bølgende sletteformer, slake skråninger og bakker. Særlig langs særlig vestsida av Mjøsa munner flere mindre ”hengende” daler ut.