Decrease in Brain Serotonin 2 Receptor Binding In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

United States Patent (19) 11 Patent Number: 5,902,815 Olney Et Al
USOO5902815A United States Patent (19) 11 Patent Number: 5,902,815 Olney et al. (45) Date of Patent: May 11, 1999 54 USE OF 5HT2A SEROTONIN AGONISTS TO Hougaku, H. et al., “Therapeutic effect of lisuride maleate on PREVENT ADVERSE EFFECTS OF NMDA post-stroke depression” Nippon Ronen Igakkai ZaSShi 31: RECEPTOR HYPOFUNCTION 52-9 (1994) (abstract). Kehne, J.H. et al., “Preclinical Characterization of the Poten 75 Inventors: John W. Olney, Ladue; Nuri B. tial of the Putative Atypical Antipsychotic MDL 100,907 as Farber, University City, both of Mo. a Potent 5-HT2A Antagonist with a Favorable CNS Saftey Profile.” The Journal of Pharmacology and Experimental 73 Assignee: Washington University, St. Louis, Mo. Therapuetics 277: 968–981 (1996). Maurel-Remy, S. et al., “Blockade of phencyclidine-induced 21 Appl. No.: 08/709,222 hyperlocomotion by clozapine and MDL 100,907 in rats reflects antagonism of 5-HT2A receptors' European Jour 22 Filed: Sep. 3, 1996 nal of Pharmacology 280: R9–R11 (1995). 51) Int. Cl. ........................ A61K 31/445; A61K 31/54; Olney, J.W., et al., “NMDAantagonist neurotoxicity: Mecha A61K 31/135 nism and prevention,” Science 254: 1515–1518 (1991). 52 U.S. Cl. .......................... 514/285; 514/315; 514/318; Olney, J.W., et al., “Glutamate receptor dysfunction and 514/646 schizophrenia.” Arch. Gen. Psychiatry 52:998-1007 (1995). 58 Field of Search ............................. 514/285; 314/315, Pulvirenti, L. et al., “Dopamine receptor agonists, partial 314/318, 646 agonists and psychostimulant addiction' Trends Pharmacol Sci 15: 374-9 (1994). 56) References Cited Robles, R.G. et al., “Natriuretic Effects of Dopamine Agonist Drugs in Models of Reduced Renal Mass” Journal of U.S. -

Fushi-Tarazu During Segmentation
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 5441-5445, June 1995 Developmental Biology Drosophila 5-HT2 serotonin receptor: Coexpression with fushi-tarazu during segmentation (in situ hybridization/G protein-coupled receptors/pair-rule gene) JEAN-FRAN;OIS CoLAs*, JEAN-MARIE LAUNAYt, ODILE KELLERMANNt, PHILIPPE ROSAY*, AND Luc MAROTEAUX*§ *Institut de Genetique et de Biologie Mol6culaire et Cellulaire, Centre National de la Recherche Scientifique, Institut National de la Sante et de la Recherche Medicale, Universite de Strasbourg, BP 163, 67404 Illkirch Cedex, France; tH6pital Lariboisiere, Service de Biochimie, 2 rue Ambroise Pare, 75475 Paris Cedex 10, France; and tDepartement de Biologie Moleculaire, Institut Pasteur, 25 rue du Dr Roux, 75724 Paris Cedex 15, France Communicated by Walter J. Gehring, University ofBasel, Basel, Switzerland, March 6, 1995 (received for review December 5, 1994) ABSTRACT Serotonin, first described as a neurotrans- ergic neurons (6), and to incomplete sclerotization of the mitter in invertebrates, has been investigated mostly for its cuticule (3). functions in the mature central nervous system of higher We report the characterization of a Drosophila serotonin vertebrates. Serotonin receptor diversity has been described receptor that displays a typical 5-HT2 receptor sequence, gene in the mammalian brain and in insects. We report the isolation organization, and pharmacology.5 It is expressed in the central of a cDNA coding for a Drosophila melanogaster serotonin nervous system (CNS) during larval and adult stages. More receptor that displays a sequence, a gene organization, and surprisingly, this receptor is expressed at the blastoderm stage pharmacological properties typical of the mammalian 5-HT2 of embryogenesis in a pattern similar to that of the pair-rule serotonin receptor subtype. -

Inhaled Loxapine Monograph
Inhaled Loxapine Monograph Inhaled Loxapine (ADASUVE) National Drug Monograph February 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechanism of Inhaled loxapine is a typical antipsychotic used in the treatment of acute Action agitation associated with schizophrenia and bipolar I disorder in adults. Loxapine’s mechanism of action for reducing agitation in schizophrenia and bipolar I disorder is unknown. Its effects are thought to be mediated through blocking postsynaptic dopamine D2 receptors as well as some activity at the serotonin 5-HT2A receptors. Indication(s) Under Review in Inhaled loxapine is a typical antipsychotic indicated for the acute treatment of this document (may include agitation associated with schizophrenia or bipolar I disorder in adults. off label) Off-label use Agitation related to any other cause not due to schizophrenia and bipolar I disorder. Dosage Form(s) Under 10mg oral inhalation using a new STACCATO inhaler device. Review REMS REMS No REMS Post-marketing Study Required See Other Considerations for additional REMS information Pregnancy Rating C Executive Summary Efficacy Inhaled loxapine was superior to placebo in reducing acute agitation at 2 hours post dose measured by the Positive and Negative Syndrome Scale-Excited Component (PEC) in patients with bipolar I disorder and schizophrenia. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (Also Belong to Psychiatric Medication Category) • Codeine (In 222® Tablets, Tylenol® No
CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (also belong to psychiatric medication category) • codeine (in 222® Tablets, Tylenol® No. 1/2/3/4, Fiorinal® C, Benzodiazepines Codeine Contin, etc.) • heroin • alprazolam (Xanax®) • hydrocodone (Hycodan®, etc.) • chlordiazepoxide (Librium®) • hydromorphone (Dilaudid®) • clonazepam (Rivotril®) • methadone • diazepam (Valium®) • morphine (MS Contin®, M-Eslon®, Kadian®, Statex®, etc.) • flurazepam (Dalmane®) • oxycodone (in Oxycocet®, Percocet®, Percodan®, OxyContin®, etc.) • lorazepam (Ativan®) • pentazocine (Talwin®) • nitrazepam (Mogadon®) • oxazepam ( Serax®) Alcohol • temazepam (Restoril®) Inhalants Barbiturates • gases (e.g. nitrous oxide, “laughing gas”, chloroform, halothane, • butalbital (in Fiorinal®) ether) • secobarbital (Seconal®) • volatile solvents (benzene, toluene, xylene, acetone, naptha and hexane) Buspirone (Buspar®) • nitrites (amyl nitrite, butyl nitrite and cyclohexyl nitrite – also known as “poppers”) Non-Benzodiazepine Hypnotics (also belong to psychiatric medication category) • chloral hydrate • zopiclone (Imovane®) Other • GHB (gamma-hydroxybutyrate) • Rohypnol (flunitrazepam) CENTRAL NERVOUS SYSTEM STIMULANTS Amphetamines Caffeine • dextroamphetamine (Dexadrine®) Methelynedioxyamphetamine (MDA) • methamphetamine (“Crystal meth”) (also has hallucinogenic actions) • methylphenidate (Biphentin®, Concerta®, Ritalin®) • mixed amphetamine salts (Adderall XR®) 3,4-Methelynedioxymethamphetamine (MDMA, Ecstasy) (also has hallucinogenic actions) Cocaine/Crack -

A New Framework for Investigating Antipsychotic Action in Humans: Lessons from PET Imaging S Kapur
Molecular Psychiatry (1998) 3, 135–140 1998 Stockton Press All rights reserved 1359–4184/98 $12.00 PERSPECTIVE A new framework for investigating antipsychotic action in humans: lessons from PET imaging S Kapur Schizophrenia Division and the PET Centre, The Clarke Institute of Psychiatry, Department of Psychiatry, University of Toronto; Rotman Research Institute, Baycrest Centre, University of Toronto With a decade of neuroreceptor imaging of antipsychotics behind us, this article attempts to synthesise what has been learnt about the mechanism of action of antipsychotics using these techniques. The data show that: (i) the ‘typical’ antipsychotics bind mainly to the dopamine D2 receptor, and that 60–80% D2 occupancy may provide optimal antipsychotic response with little extrapyramidal side effects; (ii) all the clinically available ‘atypical’ antipsychotics show a higher occupancy of the 5-HT2 than D2 receptors; (iii) however, these ‘atypical’ antipsy- chotics differ in their D2 occupancy. The D2 occupancy of risperidone is within the typical range (ie Ͼ 60%) while that of clozapine is clearly lower (Ͻ60%); (iv) antipsychotics with com- bined 5-HT2/D2 antagonism lose some of their ‘atypical’ properties if used in doses where Ͼ their D2 occupancy is too high ( 80%). Based on these data a framework is suggested wherein antipsychotics may be classified on the basis of their D2 and 5-HT2 occupancy in patients at steady state while taking clinically relevant doses. Within this framework typical antipsy- chotics are classified as ‘high-D2’, risperidone as ‘high-D2 high-5HT2’ and clozapine as a ‘low- D2 high-5HT2’ antipsychotic. The justification, limitations and the value of this framework in understanding and investigating newer antipsychotics is discussed. -

Schizophrenia Or Bipolar Disorder
Appendix D- NICE’s response to consultee and commentator comments on the draft scope and provisional matrix National Institute for Health and Clinical Excellence Proposed Single Technology Appraisal (STA) Loxapine inhalation for the treatment of acute agitation and disturbed behaviours associated with schizophrenia or bipolar disorder Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Comment 1: the draft remit Section Consultees Comments Action Appropriateness Alexza Yes as this topic is relevant to New Horizons: A Shared Vision for Mental Health Comment noted. No Pharmaceuticals (2009)1 and to The National Service Framework for Mental Health: Modern action required. Standards and Service Models (1999).2 Royal College of The appraisal of use of alternative matrices for drug delivery is important. It is Comment noted. No Pathologists particularly important in situation where there may be difficulties in administering action required. traditional drugs via intramuscular or intravenous routes. Therefore this worthy of NICE appraisal. Royal College of It is appropriate to consider novel methods to administer medications for this Comment noted. No Nursing condition. action required. Wording Alexza Revise to: Comment noted. Pharmaceuticals To appraise the clinical and cost effectiveness of Staccato® loxapine within the Consultees agreed that licensed indication for the rapid control of agitation and disturbed behaviors in the draft remit should patients with schizophrenia or in patients with bipolar -

Name and Address of the Sponsor and Monitor
PROTOCOL SCRIPT PROTOCOL FULL TITLE: A randomised, balanced, double-blind two-way crossover design study to evaluate the effects of SRC kinase inhibitor, Saracatinib, on brain activity associated with visual processing in patients with Parkinson’s disease Psychosis. Protocol Short Title/Acronym: SRC inhibition as a potential target for Parkinson’s disease psychosis- SCRIPT Trial Identifiers REC Number – [Awaiting further information] Co-Sponsors: King’s College London & Kings College Hospital NHS trust Chief Investigator Name: Professor Mitul Mehta Address: Department of Neuroimaging, Institute of Psychology, Psychiatry and Neuroscience, King’s College London, London SE5 8AF Telephone: +44 (0) 2032283053 Email: [email protected] Name and address of Co-Investigator(s), Statistician, Laboratories etc Name: Dr Dominic ffytche Address: Department of Old Age Psychiatry, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF Telephone: Fax: Protocol Version 2.2 23/08//2018 Page 1 of 49 IRAS ID: 247303 PROTOCOL SCRIPT Email: [email protected] Name: Professor K Ray Chaudhuri Address: Dept. Basic and Clinical Neuroscience, The Maurice Wohl Clinical Neuroscience Institute, King's College London, Cutcombe Road, London SE5 9RT Telephone: Fax: Email: [email protected] Name: Professor Dag Aarsland Address: Department of Old Age Psychiatry, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF Telephone: Fax: Email: [email protected] Protocol Version 2.2 23/08//2018 Page 2 of 49 IRAS ID: 247303 PROTOCOL SCRIPT 1. Study Synopsis Title of clinical trial A randomised, balanced, double-blind two-way crossover design study to evaluate the effects of SRC kinase inhibitor, Saracatinib, on brain activity associated with visual processing in patients with Parkinson’s disease Psychosis. -

Antipsychotics
HEALTH PARTNERS PLANS PRIOR AUTHORIZATION REQUEST FORM Antipsychotics Phone: 215-991-4300 Fax back to: 866-240-3712 Health Partners Plans manages the pharmacy drug benefit for your patient. Certain requests for coverage require review with the prescribing physician. Please answer the following questions and fax this form to the number listed above. PLEASE NOTE: Any information (patient, prescriber, drug, labs) left blank, illegible, or not attached WILL DELAY the review process. Patient Name: Prescriber Name: HPP Member Number: Fax: Phone: Date of Birth: Office Contact: Patient Primary Phone: NPI: PA PROMISe ID: Address: Address: City, State ZIP: City, State ZIP: Line of Business: Medicaid CHIP Specialty Pharmacy (if applicable): Drug Name: Strength: Quantity: Refills: Directions: Diagnosis Code: Diagnosis: HPP’s maximum approval time is 12 months but may be less depending on the drug. Please attach any pertinent medical history including labs and information for this member that may support approval. Please answer the following questions and sign. Q1. Is this a request for a preferred antipsychotic drug (e.g., Abilify Maintena, aripiprazole tablet, Aristada, Aristada Initio, clozapine tablet, fluphenazine tablet, fluphenazine decanoate injection, Haldol 5 mg ampule, haloperidol tablet, haloperidol decanoate injection, haloperidol lactate injection, haloperidol lactate oral concentrate, Invega Sustenna, Invega Trinza, loxapine capsule, olanzapine tablet, perphenazine tablet, Perseris, quetiapine extended-release, quetiapine immediate-release, -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -
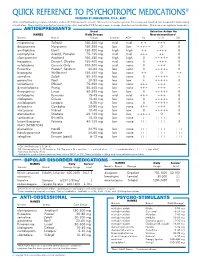
Quick Reference to Psychotropic Medications®
QUICK REFERENCE TO PSYCHOTROPIC MEDICATIONS ® DEVELOPED BY JOHN PRESTON, PSY.D., ABPP To the best of our knowledge recommended doses and side effects listed below are accurate. However, this is meant as a general reference only, and should not serve as a guideline for prescribing of medications. Please check the manufacturer’s product information sheet or the P.D.R. for any changes in dosage schedule or contraindications. (Brand names are registered trademarks.) ANTIDEPRESSANTS Usual Selective Action On NAMES Daily Dosage Neurotransmitters2 Generic Brand Range Sedation ACH 1 NE 5-HT DA imipramine Tofranil 150-300 mg mid mid + + +++ 0 desipramine Norpramin 150-300 mg low low +++++ 0 0 amitriptyline Elavil 150-300 mg high high ++ ++++ 0 nortriptyline Aventyl, Pamelor 75-125 mg mid mid +++ ++ 0 clomipramine Anafranil 150-250 mg high high 0 +++++ 0 trazodone Desyrel, Oleptro 150-400 mg mid none 0 ++++ 0 nefazodone Generic Only 100-300 mg mid none 0 +++ 0 ÁXR[HWLQH 3UR]DF 4, Sarafem 20-80 mg low none 0 +++++ 0 bupropion Wellbutrin 4 150-400 mg low none ++ 0 ++ sertraline Zoloft 50-200 mg low none 0 +++++ + SDUR[HWLQH 3D[LO PJ ORZ ORZ YHQODID[LQH (IIH[RU 4 75-350 mg low none +++ +++ + GHVYHQODID[LQH 3ULVWLT PJ ORZ QRQH ÁXYR[DPLQH /XYR[ PJ ORZ ORZ mirtazapine Remeron 15-45 mg mid mid +++ +++ 0 FLWDORSUDP &HOH[D PJ ORZ QRQH HVFLWDORSUDP /H[DSUR PJ ORZ QRQH GXOR[HWLQH &\PEDOWD PJ ORZ QRQH vilazodone Viibryd 10-40 mg low low 0 +++++ 0 DWRPR[HWLQH 6WUDWWHUD PJ ORZ ORZ YRUWLR[HWLQH %ULQWHOOL[ PJ ORZ QRQH levomilnacipran Fetzima