Inhaled Loxapine Monograph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differentiating Between Anxiety, Syncope & Anaphylaxis
Differentiating between anxiety, syncope & anaphylaxis Dr. Réka Gustafson Medical Health Officer Vancouver Coastal Health Introduction Anaphylaxis is a rare but much feared side-effect of vaccination. Most vaccine providers will never see a case of true anaphylaxis due to vaccination, but need to be prepared to diagnose and respond to this medical emergency. Since anaphylaxis is so rare, most of us rely on guidelines to assist us in assessment and response. Due to the highly variable presentation, and absence of clinical trials, guidelines are by necessity often vague and very conservative. Guidelines are no substitute for good clinical judgment. Anaphylaxis Guidelines • “Anaphylaxis is a potentially life-threatening IgE mediated allergic reaction” – How many people die or have died from anaphylaxis after immunization? Can we predict who is likely to die from anaphylaxis? • “Anaphylaxis is one of the rarer events reported in the post-marketing surveillance” – How rare? Will I or my colleagues ever see a case? • “Changes develop over several minutes” – What is “several”? 1, 2, 10, 20 minutes? • “Even when there are mild symptoms initially, there is a potential for progression to a severe and even irreversible outcome” – Do I park my clinical judgment at the door? What do I look for in my clinical assessment? • “Fatalities during anaphylaxis usually result from delayed administration of epinephrine and from severe cardiac and respiratory complications. “ – What is delayed? How much time do I have? What is anaphylaxis? •an acute, potentially -

(Loxapine) REMS
Current as of 6/1/2013.® This document may not be part of the latest approved REMS. Alexza Pharmaceuticals, Inc. 2091 Stierlin Court, Mountain View, CA 94043 IMPORTANT DRUG WARNING Risk of bronchospasm with ADASUVE™ (loxapine) inhalation powder ADASUVE™ available only in enrolled healthcare facilities, under an FDA-required REMS Program Dear Healthcare Professional: This letter contains important safety information for ADASUVE™ (loxapine) Inhalation Powder. ADASUVE is a drug-device combination product that delivers the antipsychotic, loxapine, by oral inhalation. ADASUVE has been approved by the US Food and Drug Administration (FDA) for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. FDA has determined that a Risk Evaluation and Mitigation Strategy (REMS) is necessary to ensure that the benefits of treatment outweigh the risk of bronchospasm in patients treated with ADASUVE. ADASUVE is not available in an outpatient setting. If you are an outpatient provider, you are receiving this letter because ADASUVE may be, or may have been, administered to one of your patients in an enrolled healthcare facility. Enrolled healthcare facilities, such as an acute care or inpatient setting, must have immediate on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management, eg, intubation and mechanical ventilation. (See below for more facility enrollment requirements.) It is important that anyone caring for patients treated with ADASUVE be aware of the risk of bronchospasm after administration. This letter does not contain a complete list of all the risks associated with ADASUVE. Reference ID:Please 3235446 see the enclosed full prescribing information for more information. -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Beta Adrenergic Agents
Beta2 Adrenergic Agents –Long Acting Review 04/10/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Comments and suggestions may be sent to [email protected]. -

Decrease in Brain Serotonin 2 Receptor Binding In
ORIGINAL ARTICLE Decrease in Brain Serotonin 2 Receptor Binding in Patients With Major Depression Following Desipramine Treatment A Positron Emission Tomography Study With Fluorine-18–Labeled Setoperone Lakshmi N. Yatham, MBBS, FRCPC; Peter F. Liddle, PhD, MBBS; Joelle Dennie, MSc; I-Shin Shiah, MD; Michael J. Adam, PhD; Carol J. Lane, MSc; Raymond W. Lam, MD; Thomas J. Ruth, PhD Background: The neuroreceptor changes involved in Results: Eight of the 10 patients responded to desipra- therapeutic efficacy of various antidepressants remain un- mine treatment as indicated by more than 50% decrease clear. Preclinical studies have shown that long-term ad- in Hamilton Depression Rating Scale scores. Depressed ministration of various antidepressants causes down- patients showed a significant decrease in 5-HT2 recep- regulation of brain serotonin 2 (5-HT2) receptors in rodents, tor binding as measured by setoperone binding in fron- but it is unknown if similar changes occur following anti- tal, temporal, parietal, and occipital cortical regions fol- depressant treatment in depressed patients. Our purpose, lowing desipramine treatment. The decrease in 5-HT2 therefore, was to assess the effects of treatment with desi- receptor binding was observed bilaterally and was par- pramine hydrochloride on brain 5-HT2 receptors in de- ticularly prominent in frontal cortex. pressed patients using positron emission tomography (PET) and fluorine-18 (18F)–labeled setoperone. Conclusions: Depressed patients showed a significant reduction in available 5-HT2 receptors in the brain fol- Methods: Eleven patients who met DSM-IV criteria for lowing desipramine treatment, but it is unknown if this major depression as determined by a structured clinical change in 5-HT2 receptors is due to clinical improve- interview for DSM-III-R diagnosis and suitable for treat- ment or an effect of desipramine that is unrelated to clini- ment with desipramine were recruited. -

CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (Also Belong to Psychiatric Medication Category) • Codeine (In 222® Tablets, Tylenol® No
CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (also belong to psychiatric medication category) • codeine (in 222® Tablets, Tylenol® No. 1/2/3/4, Fiorinal® C, Benzodiazepines Codeine Contin, etc.) • heroin • alprazolam (Xanax®) • hydrocodone (Hycodan®, etc.) • chlordiazepoxide (Librium®) • hydromorphone (Dilaudid®) • clonazepam (Rivotril®) • methadone • diazepam (Valium®) • morphine (MS Contin®, M-Eslon®, Kadian®, Statex®, etc.) • flurazepam (Dalmane®) • oxycodone (in Oxycocet®, Percocet®, Percodan®, OxyContin®, etc.) • lorazepam (Ativan®) • pentazocine (Talwin®) • nitrazepam (Mogadon®) • oxazepam ( Serax®) Alcohol • temazepam (Restoril®) Inhalants Barbiturates • gases (e.g. nitrous oxide, “laughing gas”, chloroform, halothane, • butalbital (in Fiorinal®) ether) • secobarbital (Seconal®) • volatile solvents (benzene, toluene, xylene, acetone, naptha and hexane) Buspirone (Buspar®) • nitrites (amyl nitrite, butyl nitrite and cyclohexyl nitrite – also known as “poppers”) Non-Benzodiazepine Hypnotics (also belong to psychiatric medication category) • chloral hydrate • zopiclone (Imovane®) Other • GHB (gamma-hydroxybutyrate) • Rohypnol (flunitrazepam) CENTRAL NERVOUS SYSTEM STIMULANTS Amphetamines Caffeine • dextroamphetamine (Dexadrine®) Methelynedioxyamphetamine (MDA) • methamphetamine (“Crystal meth”) (also has hallucinogenic actions) • methylphenidate (Biphentin®, Concerta®, Ritalin®) • mixed amphetamine salts (Adderall XR®) 3,4-Methelynedioxymethamphetamine (MDMA, Ecstasy) (also has hallucinogenic actions) Cocaine/Crack -

BREATHLESSNESS ABSTRACT INTRODUCTION Dyspnoea, Also
EMERGENCY MEDICINE – WHAT THE FAMILY PHYSICIAN CAN TREAT UNIT NO. 4 BREATHLESSNESS In one study of 85 patients presenting to a pulmonary unit Psychiatric conditions appropriate context of the history, physical examination, and ischaemia. Serial measurements of cardiac biomarkers are inhaler (MDI). In severe asthma, patients should be transferred breathlessness. In such cases, it is prudent to start therapies for with a complaint of chronic dyspnoea, the initial impression Psychogenic causes for acute dyspnoea is a diagnosis of the consideration of dierential diagnosis. Random testing necessary as initial results can often be normal. to ED for further treatment with nebulised ipratropium multiple conditions in the initial resuscitative phase. For Dr Pothiawala Sohil of the aetiology of dyspnoea based upon the patient history exclusion, and organic causes must be ruled out rst before without a clear dierential diagnosis will delay appropriate bromide, intravenous magnesium, ketamine, IM adrenaline, example, for a patient with a past medical history of COPD and alone was correct in only 66 percent of cases.4 us, a considering this diagnosis (e.g., panic attack).5 management. e use of dyspnoea biomarker panels does not Brain natriuretic peptide (BNP) intubation, and inhalational anaesthesia as needed. congestive cardiac failure, the initial management of sudden systematic approach, comprising of adequate history and appear to improve accuracy beyond clinical assessment and is is used to diagnose heart failure, but it can also be elevated onset of dyspnoea may include therapies directed at both these ABSTRACT diagnostic studies, and provide recommendations for initial physical examination, followed by appropriate investigations focused testing.6, 7 in uid overload secondary to renal failure. -

Adasuve, INN-Loxapine
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ADASUVE 4.5 mg inhalation powder, pre-dispensed 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each single-dose inhaler contains 5 mg loxapine and delivers 4.5 mg loxapine. 3. PHARMACEUTICAL FORM Inhalation powder, pre-dispensed (inhalation powder). White device with a mouthpiece on one end and a pull-tab protruding from the other end. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications ADASUVE is indicated for the rapid control of mild-to-moderate agitation in adult patients with schizophrenia or bipolar disorder. Patients should receive regular treatment immediately after control of acute agitation symptoms. 4.2 Posology and method of administration ADASUVE should only be administered in a hospital-setting under the supervision of a healthcare professional. Short-acting beta-agonist bronchodilator treatment should be available for treatment of possible severe respiratory side-effects (bronchospasm). Posology The recommended initial dose of ADASUVE is 9.1 mg. A second dose can be given after 2 hours, if necessary. No more than two doses should be administered. A lower dose of 4.5 mg may be given if the 9.1 mg dose was not previously tolerated by the patient or if the physician decides a lower dose is more appropriate. Patient should be observed during the first hour after each dose for signs and symptoms of bronchospasm. Elderly The safety and efficacy of ADASUVE in patients older than 65 years of age have not been established. No data are available. Renal and/or hepatic impairment ADASUVE has not been studied in patients with renal or hepatic impairment. -

Schizophrenia Or Bipolar Disorder
Appendix D- NICE’s response to consultee and commentator comments on the draft scope and provisional matrix National Institute for Health and Clinical Excellence Proposed Single Technology Appraisal (STA) Loxapine inhalation for the treatment of acute agitation and disturbed behaviours associated with schizophrenia or bipolar disorder Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Comment 1: the draft remit Section Consultees Comments Action Appropriateness Alexza Yes as this topic is relevant to New Horizons: A Shared Vision for Mental Health Comment noted. No Pharmaceuticals (2009)1 and to The National Service Framework for Mental Health: Modern action required. Standards and Service Models (1999).2 Royal College of The appraisal of use of alternative matrices for drug delivery is important. It is Comment noted. No Pathologists particularly important in situation where there may be difficulties in administering action required. traditional drugs via intramuscular or intravenous routes. Therefore this worthy of NICE appraisal. Royal College of It is appropriate to consider novel methods to administer medications for this Comment noted. No Nursing condition. action required. Wording Alexza Revise to: Comment noted. Pharmaceuticals To appraise the clinical and cost effectiveness of Staccato® loxapine within the Consultees agreed that licensed indication for the rapid control of agitation and disturbed behaviors in the draft remit should patients with schizophrenia or in patients with bipolar -

Antipsychotics
HEALTH PARTNERS PLANS PRIOR AUTHORIZATION REQUEST FORM Antipsychotics Phone: 215-991-4300 Fax back to: 866-240-3712 Health Partners Plans manages the pharmacy drug benefit for your patient. Certain requests for coverage require review with the prescribing physician. Please answer the following questions and fax this form to the number listed above. PLEASE NOTE: Any information (patient, prescriber, drug, labs) left blank, illegible, or not attached WILL DELAY the review process. Patient Name: Prescriber Name: HPP Member Number: Fax: Phone: Date of Birth: Office Contact: Patient Primary Phone: NPI: PA PROMISe ID: Address: Address: City, State ZIP: City, State ZIP: Line of Business: Medicaid CHIP Specialty Pharmacy (if applicable): Drug Name: Strength: Quantity: Refills: Directions: Diagnosis Code: Diagnosis: HPP’s maximum approval time is 12 months but may be less depending on the drug. Please attach any pertinent medical history including labs and information for this member that may support approval. Please answer the following questions and sign. Q1. Is this a request for a preferred antipsychotic drug (e.g., Abilify Maintena, aripiprazole tablet, Aristada, Aristada Initio, clozapine tablet, fluphenazine tablet, fluphenazine decanoate injection, Haldol 5 mg ampule, haloperidol tablet, haloperidol decanoate injection, haloperidol lactate injection, haloperidol lactate oral concentrate, Invega Sustenna, Invega Trinza, loxapine capsule, olanzapine tablet, perphenazine tablet, Perseris, quetiapine extended-release, quetiapine immediate-release, -
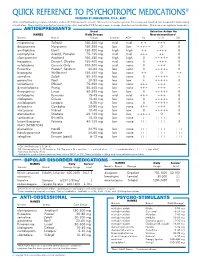
Quick Reference to Psychotropic Medications®
QUICK REFERENCE TO PSYCHOTROPIC MEDICATIONS ® DEVELOPED BY JOHN PRESTON, PSY.D., ABPP To the best of our knowledge recommended doses and side effects listed below are accurate. However, this is meant as a general reference only, and should not serve as a guideline for prescribing of medications. Please check the manufacturer’s product information sheet or the P.D.R. for any changes in dosage schedule or contraindications. (Brand names are registered trademarks.) ANTIDEPRESSANTS Usual Selective Action On NAMES Daily Dosage Neurotransmitters2 Generic Brand Range Sedation ACH 1 NE 5-HT DA imipramine Tofranil 150-300 mg mid mid + + +++ 0 desipramine Norpramin 150-300 mg low low +++++ 0 0 amitriptyline Elavil 150-300 mg high high ++ ++++ 0 nortriptyline Aventyl, Pamelor 75-125 mg mid mid +++ ++ 0 clomipramine Anafranil 150-250 mg high high 0 +++++ 0 trazodone Desyrel, Oleptro 150-400 mg mid none 0 ++++ 0 nefazodone Generic Only 100-300 mg mid none 0 +++ 0 ÁXR[HWLQH 3UR]DF 4, Sarafem 20-80 mg low none 0 +++++ 0 bupropion Wellbutrin 4 150-400 mg low none ++ 0 ++ sertraline Zoloft 50-200 mg low none 0 +++++ + SDUR[HWLQH 3D[LO PJ ORZ ORZ YHQODID[LQH (IIH[RU 4 75-350 mg low none +++ +++ + GHVYHQODID[LQH 3ULVWLT PJ ORZ QRQH ÁXYR[DPLQH /XYR[ PJ ORZ ORZ mirtazapine Remeron 15-45 mg mid mid +++ +++ 0 FLWDORSUDP &HOH[D PJ ORZ QRQH HVFLWDORSUDP /H[DSUR PJ ORZ QRQH GXOR[HWLQH &\PEDOWD PJ ORZ QRQH vilazodone Viibryd 10-40 mg low low 0 +++++ 0 DWRPR[HWLQH 6WUDWWHUD PJ ORZ ORZ YRUWLR[HWLQH %ULQWHOOL[ PJ ORZ QRQH levomilnacipran Fetzima -

Adasuve, INN-Loxapine
13 December 2012 EMA/91055/2013 Committee for Medicinal Products for Human Use (CHMP) Assessment report Adasuve International non-proprietary name: loxapine Procedure No. EMEA/H/C/002400 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7523 7455 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Contents 1. Background information on the procedure .............................................. 8 1.1. Submission of the dossier .................................................................................... 8 Information on Paediatric requirements ....................................................................... 8 Information relating to orphan market exclusivity .......................................................... 8 Scientific Advice ....................................................................................................... 8 Licensing status ....................................................................................................... 8 1.2. Steps taken for the assessment of the product ....................................................... 9 2. Scientific discussion .............................................................................. 10 2.1. Introduction ...................................................................................................