Respiratory Distress/Bronchospasm for COPD/Asthma Exacerbations and Any Bronchospasms/Wheezing Not from Pulmonary Edema
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differentiating Between Anxiety, Syncope & Anaphylaxis
Differentiating between anxiety, syncope & anaphylaxis Dr. Réka Gustafson Medical Health Officer Vancouver Coastal Health Introduction Anaphylaxis is a rare but much feared side-effect of vaccination. Most vaccine providers will never see a case of true anaphylaxis due to vaccination, but need to be prepared to diagnose and respond to this medical emergency. Since anaphylaxis is so rare, most of us rely on guidelines to assist us in assessment and response. Due to the highly variable presentation, and absence of clinical trials, guidelines are by necessity often vague and very conservative. Guidelines are no substitute for good clinical judgment. Anaphylaxis Guidelines • “Anaphylaxis is a potentially life-threatening IgE mediated allergic reaction” – How many people die or have died from anaphylaxis after immunization? Can we predict who is likely to die from anaphylaxis? • “Anaphylaxis is one of the rarer events reported in the post-marketing surveillance” – How rare? Will I or my colleagues ever see a case? • “Changes develop over several minutes” – What is “several”? 1, 2, 10, 20 minutes? • “Even when there are mild symptoms initially, there is a potential for progression to a severe and even irreversible outcome” – Do I park my clinical judgment at the door? What do I look for in my clinical assessment? • “Fatalities during anaphylaxis usually result from delayed administration of epinephrine and from severe cardiac and respiratory complications. “ – What is delayed? How much time do I have? What is anaphylaxis? •an acute, potentially -

(Loxapine) REMS
Current as of 6/1/2013.® This document may not be part of the latest approved REMS. Alexza Pharmaceuticals, Inc. 2091 Stierlin Court, Mountain View, CA 94043 IMPORTANT DRUG WARNING Risk of bronchospasm with ADASUVE™ (loxapine) inhalation powder ADASUVE™ available only in enrolled healthcare facilities, under an FDA-required REMS Program Dear Healthcare Professional: This letter contains important safety information for ADASUVE™ (loxapine) Inhalation Powder. ADASUVE is a drug-device combination product that delivers the antipsychotic, loxapine, by oral inhalation. ADASUVE has been approved by the US Food and Drug Administration (FDA) for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. FDA has determined that a Risk Evaluation and Mitigation Strategy (REMS) is necessary to ensure that the benefits of treatment outweigh the risk of bronchospasm in patients treated with ADASUVE. ADASUVE is not available in an outpatient setting. If you are an outpatient provider, you are receiving this letter because ADASUVE may be, or may have been, administered to one of your patients in an enrolled healthcare facility. Enrolled healthcare facilities, such as an acute care or inpatient setting, must have immediate on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management, eg, intubation and mechanical ventilation. (See below for more facility enrollment requirements.) It is important that anyone caring for patients treated with ADASUVE be aware of the risk of bronchospasm after administration. This letter does not contain a complete list of all the risks associated with ADASUVE. Reference ID:Please 3235446 see the enclosed full prescribing information for more information. -

Inhaled Loxapine Monograph
Inhaled Loxapine Monograph Inhaled Loxapine (ADASUVE) National Drug Monograph February 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechanism of Inhaled loxapine is a typical antipsychotic used in the treatment of acute Action agitation associated with schizophrenia and bipolar I disorder in adults. Loxapine’s mechanism of action for reducing agitation in schizophrenia and bipolar I disorder is unknown. Its effects are thought to be mediated through blocking postsynaptic dopamine D2 receptors as well as some activity at the serotonin 5-HT2A receptors. Indication(s) Under Review in Inhaled loxapine is a typical antipsychotic indicated for the acute treatment of this document (may include agitation associated with schizophrenia or bipolar I disorder in adults. off label) Off-label use Agitation related to any other cause not due to schizophrenia and bipolar I disorder. Dosage Form(s) Under 10mg oral inhalation using a new STACCATO inhaler device. Review REMS REMS No REMS Post-marketing Study Required See Other Considerations for additional REMS information Pregnancy Rating C Executive Summary Efficacy Inhaled loxapine was superior to placebo in reducing acute agitation at 2 hours post dose measured by the Positive and Negative Syndrome Scale-Excited Component (PEC) in patients with bipolar I disorder and schizophrenia. -

Hypercapnia in Hemodialysis (HD)
ISSN: 2692-532X DOI: 10.33552/AUN.2020.01.000508 Annals of Urology & Nephrology Mini Review Copyright © All rights are reserved by David Tovbin Hypercapnia in Hemodialysis (HD) David Tovbin* Department of Nephrology, Emek Medical Center, Israel *Corresponding author: David Tovbin, Department of Nephrology, Emek Medical Received Date: February 04, 2019 Center, Afula, Israel. Published Date: February 14, 2019 Introduction 2 case reports and in our experience with similar patients, BiPAP Acute intra-dialytic exacerbation of hypercapnia in hemodialysis prevented intra-dialytic exacerbation of hypercapnia and possibly (HD) patient has been initially reported 18 years ago [1]. Subsequent respiratory arrest [1,2]. In recent years, new interest was raised similar case was reported few years later [2]. Common features of to HD dialysate bicarbonate concentration. After standardizing to both patients were morbid obesity, a previously stable HD sessions and an acute respiratory infection at time of hypercapnia [1,2]. HD pre-dialysis serum bicarbonate level was recommended as >22 patients with decreased ventilation reserve, due to morbid obesity inflammation malnutrition complex and comorbidities midweek mEq/L [11]. As higher dialysate bicarbonate concentration became with or without obstructive sleep apnea (OSA) and/or obesity more prevalent, a large observation cohort study demonstrated hypoventilation syndrome (OHS) as well as chronic obstructive that high dialysate bicarbonate concentration was associated pulmonary disease (COPD), are at increased risk. COPD is common with worse outcome especially in the more acidotic patients among HD patients but frequently under-diagnosed [3]. Most [12]. However, still not enough attention is paid to HD dialysate COPD patients do well during HD with only mild- moderate pCO2 bicarbonate in the increasing number of patients with impaired increases and slightly decreased pH as compared to non-COPD ventilation, and to their risk of intra-dialytic exacerbation of chronic HD patients [2,4]. -

Basic Life Support Health Care Provider
ELLIS & ASSOCIATES Health Care Provider Basic Life Support MEETS CURRENT CPR & ECC GUIDELINES Ellis & Associates / Safety & Health HEALTH CARE PROVIDER BASIC LIFE SUPPORT - I Ellis & Associates, Inc. P.O. Box 2160, Windermere, FL 34786-2160 www.jellis.com Copyright © 2016 by Ellis & Associates, LLC All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the publisher, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law. For permission requests, write to the publisher, addressed “Attention: Permissions Coordinator,” at the address below. Ellis & Associates P.O. Box 2160, Windermere, FL 34786-2160 Ordering Information: Quantity sales. Special discounts are available on quantity purchases by corporations, associations, trade bookstores and wholesalers. For details, contact the publisher at the address above. Disclaimer: The procedures and protocols presented in this manual and the course are based on the most current recommendations of responsible medical sources, including the International Liaison Committee on Resuscitation (ILCOR) 2015 Guidelines for CPR & ECC. Ellis & Associates, however, make no guarantee as to, and assume no responsibility for, the correctness, sufficiency, or completeness of such recommendations or information. Additional procedures may be required under particular circumstances. Ellis & Associates disclaims all liability for damages of any kind arising from the use of, reference to, reliance on, or performance based on such information. Library of Congress Cataloging-in-Publication Data Not Available at Time of Printing ISBN 978-0-9961108-0-8 Unless otherwise indicated on the Credits Page, all photographs and illustrations are copyright protected by Ellis & Associates. -

Appendix A: Codes Used to Define Principal Diagnosis of COVID-19, Sepsis, Or Respiratory Disease
Appendix A: Codes used to define principal diagnosis of COVID-19, sepsis, or respiratory disease ICD-10 code Description N J21.9 'Acute bronchiolitis, unspecified' 1 J80 'Acute respiratory distress syndrome' 2 J96.01 'Acute respiratory failure with hypoxia' 15 J06.9 'Acute upper respiratory infection, unspecified' 7 U07.1 'COVID-19' 1998 J44.1 'Chronic obstructive pulmonary disease with (acute) exacerbation' 1 R05 'Cough' 1 O99.52 'Diseases of the respiratory system complicating childbirth' 3 O99.512 'Diseases of the respiratory system complicating pregnancy, second trimester' 1 J45.41 'Moderate persistent asthma with (acute) exacerbation' 2 B97.29 'Other coronavirus as the cause of diseases classified elsewhere' 33 J98.8 'Other specified respiratory disorders' 16 A41.89 'Other specified sepsis' 1547 J12.89 'Other viral pneumonia' 222 J12.81 'Pneumonia due to SARS-associated coronavirus' 2 J18.9 'Pneumonia, unspecified organism' 8 R09.2 'Respiratory arrest' 1 J96.91 'Respiratory failure, unspecified with hypoxia' 1 A41.9 'Sepsis, unspecified organism' 155 R06.02 'Shortness of breath' 1 J22 'Unspecified acute lower respiratory infection' 4 J12.9 'Viral pneumonia, unspecified' 6 Appendix B: Average marginal effect by month, main analysis and sensitivity analyses Appendix C: Unadjusted mortality rate over time, by age group Appendix D: Results restricted to COVID-19, sepsis, or respiratory disease Any chronic Adjusted mortality Standardized Average marginal Age, median Male, Mortality, Month N condition, (95% Poisson limits) mortality ratio -

Beta Adrenergic Agents
Beta2 Adrenergic Agents –Long Acting Review 04/10/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Comments and suggestions may be sent to [email protected]. -
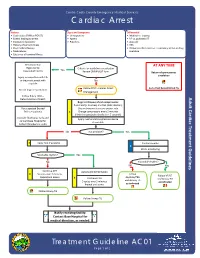
AC01 Page 1 of 2 Effective Jan
Contra Costa County Emergency Medical Services Cardiac Arrest History Signs and Symptoms Differential • Code status (DNR or POLST) • Unresponsive • Medical vs. trauma • Events leading to arrest • Apneic • VF vs. pulseless VT • Estimated downtime • Pulseless • Asystole • History of current illness • PEA • Past medical history • Primary cardiac event vs. respiratory arrest or drug • Medications overdose • Existence of terminal illness Decomposition AT ANY TIME Rigor mortis Criteria for death/no resuscitation Dependent lividity Yes Review DNR/POLST form Return of spontaneous circulation Injury incompatible with life or traumatic arrest with No asystole Follow FP09 ‐ Cardiac Arrest Go to Post Resuscitation TG Do not begin resuscitation Management Follow Policy 1004 – Determination of Death Begin continuous chest compressions Push hard (> 2 inches) and fast (100‐120/min) For suspected Excited Use metronome to ensure proper rate Delirium patients E Change compressors every 2 minutes (Limit changes/pulse checks to < 5 seconds) Consider fluid bolus early and Apply mechanical compression device contact Base Hospital for if available Sodium Bicarbonate order No ALS available? Yes E Apply AED if available Cardiac monitor P EtCO2 monitoring Shockable rhythm? Yes No Shockable rhythm? No Yes Continue CPR Automated defibrillation E 5 cycles over 2 minutes Follow Follow VF/VT Repeat and assess Asystole/PEA E Continue CPR and Airway TG and Airway TG 5 cycles over 2 minutes as indicated Repeat and assess as indicated Follow Airway TG Follow Airway TG Notify receiving facility. Contact Base Hospital for medical direction, as needed. Treatment Guideline AC01 Page 1 of 2 Effective Jan. 2016Effective Jan. 2020 Contra Costa County Emergency Medical Services Cardiac Arrest Pearls • Efforts should be directed at high quality and continuous chest compressions with limited interruptions. -

BREATHLESSNESS ABSTRACT INTRODUCTION Dyspnoea, Also
EMERGENCY MEDICINE – WHAT THE FAMILY PHYSICIAN CAN TREAT UNIT NO. 4 BREATHLESSNESS In one study of 85 patients presenting to a pulmonary unit Psychiatric conditions appropriate context of the history, physical examination, and ischaemia. Serial measurements of cardiac biomarkers are inhaler (MDI). In severe asthma, patients should be transferred breathlessness. In such cases, it is prudent to start therapies for with a complaint of chronic dyspnoea, the initial impression Psychogenic causes for acute dyspnoea is a diagnosis of the consideration of dierential diagnosis. Random testing necessary as initial results can often be normal. to ED for further treatment with nebulised ipratropium multiple conditions in the initial resuscitative phase. For Dr Pothiawala Sohil of the aetiology of dyspnoea based upon the patient history exclusion, and organic causes must be ruled out rst before without a clear dierential diagnosis will delay appropriate bromide, intravenous magnesium, ketamine, IM adrenaline, example, for a patient with a past medical history of COPD and alone was correct in only 66 percent of cases.4 us, a considering this diagnosis (e.g., panic attack).5 management. e use of dyspnoea biomarker panels does not Brain natriuretic peptide (BNP) intubation, and inhalational anaesthesia as needed. congestive cardiac failure, the initial management of sudden systematic approach, comprising of adequate history and appear to improve accuracy beyond clinical assessment and is is used to diagnose heart failure, but it can also be elevated onset of dyspnoea may include therapies directed at both these ABSTRACT diagnostic studies, and provide recommendations for initial physical examination, followed by appropriate investigations focused testing.6, 7 in uid overload secondary to renal failure. -
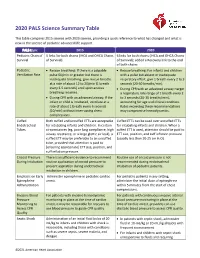
PALS Science Summary Table
2020 PALS Science Summary Table This table compares 2015 science with 2020 science, providing a quick reference to what has changed and what is new in the science of pediatric advanced life support. PALS topic 2015 2020 Pediatric Chain of 5 links for both chains (IHCA and OHCA Chains 6 links for both chains (IHCA and OHCA Chains Survival of Survival) of Survival); added a Recovery link to the end of both chains Pediatric • Rescue breathing: If there is a palpable • Rescue breathing: For infants and children Ventilation Rate pulse 60/min or greater but there is with a pulse but absent or inadequate inadequate breathing, give rescue breaths respiratory effort, give 1 breath every 2 to 3 at a rate of about 12 to 20/min (1 breath seconds (20-30 breaths/min). every 3-5 seconds) until spontaneous • During CPR with an advanced airway: target breathing resumes. a respiratory rate range of 1 breath every 2 • During CPR with an advanced airway: If the to 3 seconds (20-30 breaths/min), infant or child is intubated, ventilate at a accounting for age and clinical condition. rate of about 1 breath every 6 seconds Rates exceeding these recommendations (10/min) without interrupting chest may compromise hemodynamics. compressions. Cuffed Both cuffed and uncuffed ETTs are acceptable Cuffed ETTs can be used over uncuffed ETTs Endotracheal for intubating infants and children. In certain for intubating infants and children. When a Tubes circumstances (eg, poor lung compliance, high cuffed ETT is used, attention should be paid to airway resistance, or a large glottic air leak), a ETT size, position, and cuff inflation pressure cuffed ETT may be preferable to an uncuffed (usually less than 20-25 cm H2O). -

Adasuve, INN-Loxapine
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ADASUVE 4.5 mg inhalation powder, pre-dispensed 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each single-dose inhaler contains 5 mg loxapine and delivers 4.5 mg loxapine. 3. PHARMACEUTICAL FORM Inhalation powder, pre-dispensed (inhalation powder). White device with a mouthpiece on one end and a pull-tab protruding from the other end. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications ADASUVE is indicated for the rapid control of mild-to-moderate agitation in adult patients with schizophrenia or bipolar disorder. Patients should receive regular treatment immediately after control of acute agitation symptoms. 4.2 Posology and method of administration ADASUVE should only be administered in a hospital-setting under the supervision of a healthcare professional. Short-acting beta-agonist bronchodilator treatment should be available for treatment of possible severe respiratory side-effects (bronchospasm). Posology The recommended initial dose of ADASUVE is 9.1 mg. A second dose can be given after 2 hours, if necessary. No more than two doses should be administered. A lower dose of 4.5 mg may be given if the 9.1 mg dose was not previously tolerated by the patient or if the physician decides a lower dose is more appropriate. Patient should be observed during the first hour after each dose for signs and symptoms of bronchospasm. Elderly The safety and efficacy of ADASUVE in patients older than 65 years of age have not been established. No data are available. Renal and/or hepatic impairment ADASUVE has not been studied in patients with renal or hepatic impairment. -

CPR/AED for Professional Rescuers and Health Care Providers HANDBOOK
CPR/AED for Professional Rescuers and Health Care Providers HANDBOOK American Red Cross CPR/AED for Professional Rescuers and Health Care Providers HANDBOOK This CPR/AED for Professional Rescuers and Health Care Providers Handbook is part of the American Red Cross CPR/AED for Professional Rescuers and Health Care Providers program. By itself, it does not constitute complete and comprehensive training. Visit redcross.org to learn more about this program. The emergency care procedures outlined in this book refl ect the standard of knowledge and accepted emergency practices in the United States at the time this book was published. It is the reader’s responsibility to stay informed of changes in emergency care procedures. PLEASE READ THE FOLLOWING TERMS AND CONDITIONS BEFORE AGREEING TO ACCESS AND DOWNLOAD THE AMERICAN RED CROSS MATERIALS. BY DOWNLOADING THE MATERIALS, YOU HEREBY AGREE TO BE BOUND BY THE TERMS AND CONDITIONS. The downloadable electronic materials, including all content, graphics, images and logos, are copyrighted by and the exclusive property of The American National Red Cross (“Red Cross”). Unless otherwise indicated in writing by the Red Cross, the Red Cross grants you (“recipient”) the limited right to download, print, photocopy and use the electronic materials, subject to the following restrictions: ■ The recipient is prohibited from selling electronic versions of the materials. ■ The recipient is prohibited from revising, altering, adapting or modifying the materials. ■ The recipient is prohibited from creating any derivative works incorporating, in part or in whole, the content of the materials. ■ The recipient is prohibited from downloading the materials and putting them on their own website without Red Cross permission.