Overview of Antibody Drug Delivery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Specialty Drug List 10-22-12 Final
Iowa Medicaid Specialty Drug List Effective 10/22/2012 “Specialty” drugs include biological drugs, blood-derived products, complex molecules, and select oral, injectable, and infused medications identified by the Department and reimbursed at AWP-17% plus the dispensing fee. Specialty pricing will be applied to both the brand and generic drug products. NOTE: See the PDL at www.iowamedicaidpdl.com for specific PA criteria for the following drugs. BRAND NAME GENERIC NAME AGENTS FOR GAUCHER DISEASE ELELYSO taliglucerase alfa VPRIV velaglucerase alfa ALS RILUTEK riluzole ALCOHOL DEPENDENCE VIVITROL naltrexone AMINOGLYCOSIDES TOBI tobramycin ANTI-ASTHMATICS ALPHA PROTEINASE INHIBITORS ARALAST proteinase inhibitor ARALAST NP proteinase inhibitor PROLASTIN, C proteinase inhibitor ZEMAIRA alpha-1 proteinase inhibitor ANTIASTHMATIC - BETA - ADRENERGICS BRETHINE INJECTION turbutaline sulfate ANTIASTHMATIC - HYDRO-LYTIC ENZYMES KALYDECO ivacaftor ANTIBIOTICS - MISC. CAYSTON aztreonam lysine for inhal soln ANTI-CATAPLECTIC AGENTS XYREM sodium oxybate oral solution ANTICOAGULANTS ARIXTRA fondaparinux sodium FRAGMIN dalteparin sodium INNOHEP tinzaparin sodium LOVENOX enoxaparin sodium ANTICONVULSANTS SABRIL vigabatrin ANTIDOTES FERRIPROX deferiprone ANTIDOTES - CHELATING AGENTS CHEMET succimer EXJADE deferasirox tab for oral susp Page 1 of 10 ANTIEMETIC - TETRAHYDROCANNABINOL (THC) DERIVATIVES MARINOL dronabinol ANTIFUNGALS - ASSORTED AMBISOME amphotericin B Liposome IV for suspension ANCOBON flucytosine CANCIDAS caspofungin acetate for IV solution -

Elimination Pathways of Fusion Protein and Peptide Drugs
Review Volume 11, Issue 2, 2021, 9139 - 9147 https://doi.org/10.33263/BRIAC112.91399147 Elimination Pathways of Fusion Protein and Peptide Drugs Ali Khodadoust 1 , Hossein Aghamollaei 2 , Ali Mohammad Latifi 1 , Kazem Hasanpour 3 , Mahdi Kamali 4 , Hamid Tebyanian 5 , Gholamreza Farnoosh 1,* 1 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 2 Chemical Injuries Research Center, Systems biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran 3 Sabzevar University of Medical Sciences, School of Medicine, Sabzevar, Iran 4 Nanobiotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 5 Research Center for Prevention of Oral and Dental Diseases, Baqiyatallah University of Medical Sciences, Tehran, Iran * Correspondence: [email protected]; Scopus Author ID 55855454400 Received: 28.07.2020; Revised: 25.08.2020; Accepted: 27.08.2020; Published: 1.09.2020 Abstract: Fusion proteins have been known as an interesting subject for scientific researches in improving properties or making a new function by synergistically incorporating two protein domains into one complex. Fusion proteins are created by ligation of two or more protein domains in a single polypeptide with functional properties. Improvement of therapeutic function is one of the main goals for developing these products. Elimination from the body is one of the most important points that should be considered in the design and production of fusion proteins. This review describes some of the most important excretion/elimination pathways of fusion peptide and protein drugs as well as serious elimination challenges in the development and manufacturing of fusion proteins. Keywords: Fusion proteins; Therapy; Pharmacokinetics; Serum half-life; Peptide. -

Specialty Drug Benefit Document
Louisiana Healthcare Connections Specialty Drug Benefit ouisiana Healthcare Connections provides coverage of a number of specialty drugs. All specialty drugs, such as biopharmaceuticals and injectables, require a prior authorization (PA) to be approved for L payment by Louisiana Healthcare Connections. PA requirements are programmed specific to the drug. Since the list of specialty drugs changes over time due to new drug arrivals and other market conditions, it is important to contact Provider Services at 1-866-595-8133 or check the Louisiana Healthcare Connections website at www.LouisianaHealthConnect.com for updates to this benefit. Requests for specialty drugs can be submitted to Louisiana Healthcare Connections by filling out the Medication Prior Authorization Form that is available on the Louisiana Healthcare Connections website at www.LouisianaHealthConnect.com and faxing the request as instructed on the form. Louisiana Healthcare Connections members can receive the specialty drugs they require at any outpatient pharmacy enrolled in our pharmacy network that can supply specialty drugs. Providers that wish to have drugs distributed by a SPECIALTY PHARMACY should FAX the request to 1-866-399-0929 for review. If a provider wishes to dispense a specialty drug from OFFICE STOCK, the provider should FAX the request to Louisiana Healthcare Connections at 1-877-401-8172 for review. BRAND NAME INGREDIENTS SPECIAL INSTRUCTIONS ACTEMRA TOCILIZUMAB ACTHAR HP CORTICOTROPIN ACTIMMUNE INTERFERON GAMMA-1B ADAGEN PEGADEMASE BOVINE Limited Distribution -

MSM Chapter 1200 3/1/21
MEDICAID SERVICES MANUAL TRANSMITTAL LETTER February 23, 2021 TO: CUSTODIANS OF MEDICAID SERVICES MANUAL FROM: JESSICA KEMMERER, HIPAA PRIVACY AND CIVIL RIGHTS OFFICER /Jessica Kemmerer/ BACKGROUND AND EXPLANATION The DHCFP is proposing revisions to Medicaid Services Manual (MSM), Chapter 1200 – Prescribed Drugs, Appendix A, to reflect recommendations approved on October 22, 2020, by the Drug Use Review (DUR) Board. The proposed changes include the addition of new prior authorization criteria for Doxepine Topical, the addition of new prior authorization criteria for Zeposia® (ozanimod), addition of new prior authorization for Evenity® (romosozumab-aqqg), Prolia® (denosumab), Forteo® (teriparatide) and Tymlos® (abaloparatide) within a new combined osteoporosis agents section, and addition of new prior authorization criteria for Orilissa® (elagolix) and Oriahnn® (elagolix, estradiol, and norethindrone) within a new Gonadorpin Hormone Receptor (GnRH) Antagonist and Combinations section. Additionally, the DHCFP is proposing revisions to the existing prior authorization criteria for psychotropic medications for children and adolescents, and revision to the existing clinical criteria for Epidiolex® (cannabidiol). Throughout the chapter, grammar, punctuation and capitalization changes were made, duplications removed, acronyms used and standardized, and language reworded for clarity. Renumbering and re- arranging of sections was necessary. These changes are effective March 1, 2021. MATERIAL TRANSMITTED MATERIAL SUPERSEDED MTL N/A MTL N/A MSM Ch 1200 – Prescribed Drugs MSM Ch 1200 – Prescribed Drugs Background and Explanation of Policy Changes, Manual Section Section Title Clarifications and Updates Appendix A Psychotropic Added new policy language criteria on which specific Section N Medications for drug classes may bypass polypharmacy clinical criteria. Children and Adolescents Appendix A Reserved for Future Created a new section titled “Doxepin Topical.” Added Section W Use new prior authorization criteria for doxepin topical. -

OMONTYS® Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS---------------------- These highlights do not include all the information needed to use Dosage Form Strengths OMONTYS® safely and effectively. See full prescribing information for OMONTYS. Single use vials 2 mg/0.5 mL, 3 mg/0.5 mL, (preservative-free) 4 mg/0.5 mL, 5 mg/0.5 mL, and OMONTYS® (peginesatide) Injection, 6 mg/0.5 mL for intravenous or subcutaneous use Single use pre-filled syringes 1 mg/0.5 mL, 2 mg/0.5 mL, Initial U.S. Approval: 2012 (preservative-free) 3 mg/0.5 mL, 4 mg/0.5 mL, 5 mg/0.5 mL, and 6 mg/0.5 mL WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL Multiple use vials 10 mg/mL and 20 mg/2 mL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, (with preservative) THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE -------------------------------CONTRAINDICATIONS------------------------------ See full prescribing information for complete boxed warning. Uncontrolled hypertension (4). Chronic Kidney Disease: In controlled trials, patients experienced greater risks for -----------------------WARNINGS AND PRECAUTIONS------------------------ death, serious adverse cardiovascular reactions, and stroke Increased Mortality, Myocardial Infarction, Stroke, and when administered erythropoiesis-stimulating agents (ESAs) Thromboembolism: Using ESAs to target a hemoglobin level of to target a hemoglobin level of greater than 11 g/dL (5.1). greater than 11 g/dL increases the risk of serious adverse No trial has identified a hemoglobin target level, ESA dose, or cardiovascular reactions and has not been shown to provide dosing strategy that does not increase these risks (5.1). additional benefits (5.1). -

CUE-101, a Novel Fc Fusion Protein for Selective Targeting and Expansion of Anti-Tumor T Cells for Treatment of HPV-Driven Malignancies
CUE-101, a novel Fc fusion protein for selective targeting and expansion of anti-tumor T cells for treatment of HPV-driven malignancies Natasha Girgis1, Steven N. Quayle1, Alyssa Nelson1, Dharma Raj Thapa1, Sandrine Hulot1, Lauren Kraemer1, Miguel Moreta1, Zohra Merazga1, Robert Ruidera1, Dominic Beal1, Mark Haydock1, Jonathan Soriano1, Luke Witt1, Jessica Ryabin1, Emily Spaulding1, John F. Ross1, Saso Cemerski1, Anish Suri1, Rodolfo Chaparro1, Ronald Seidel1, Kenneth J. Pienta2, Mary C. Simcox1 1Cue Biopharma, Cambridge, Massachusetts; 2The James Buchanan Brady Urological Institute and the Department of Urology, Johns Hopkins School of Medicine, Baltimore, Maryland + + Background CUE-101 selectively expands E711-20-specific CD8 T mCUE-101 expands functional antigen-specific CD8 T • Human papilloma virus (HPV) is responsible for 72% of oropharyngeal, 90% of cervical, 90% cells from healthy human PBMCs cells in the tumor and the periphery of anal, and 71% of vulvar, vaginal, or penile cancers, causing significant morbidity and A. B. A. B. C. 9 mortality worldwide. Innovative therapies are urgently needed for these malignancies, Vehicle 100 nM CUE-101 107 15 Peripheral Blood particularly in the largely incurable metastatic setting. 106 10 5 • The E7 oncoprotein is constitutively expressed in HPV-associated cancers, is necessary for 105 ⍺PD-1 5 PE 4 Vehicle mCUE-101 Combo - 10 initiation and maintenance of malignant transformation, and is genetically conserved in 1 cancer (Mirabello 2017). 103 1 1 102 E7-Specific of CD8+ T cells % IFNg+,TNFa+ -

Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation
Review Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation Leonor S. Castro 1,†, Guilherme S. Lobo 1,†, Patrícia Pereira 2 , Mara G. Freire 1 ,Márcia C. Neves 1,* and Augusto Q. Pedro 1,* 1 CICECO–Aveiro Institute of Materials, Chemistry Department, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal; [email protected] (L.S.C.); [email protected] (G.S.L.); [email protected] (M.G.F.) 2 Centre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra, Rua Sílvio Lima-Polo II, 3030-790 Coimbra, Portugal; [email protected] * Correspondence: [email protected] (M.C.N.); [email protected] (A.Q.P.) † These authors contributed equally to this work. Abstract: The advent of biopharmaceuticals in modern medicine brought enormous benefits to the treatment of numerous human diseases and improved the well-being of many people worldwide. First introduced in the market in the early 1980s, the number of approved biopharmaceutical products has been steadily increasing, with therapeutic proteins, antibodies, and their derivatives accounting for most of the generated revenues. The success of pharmaceutical biotechnology is closely linked with remarkable developments in DNA recombinant technology, which has enabled the production of proteins with high specificity. Among promising biopharmaceuticals are interferons, first described by Isaacs and Lindenmann in 1957 and approved for clinical use in humans nearly thirty years later. Interferons are secreted autocrine and paracrine proteins, which by regulating several biochemical Citation: Castro, L.S.; Lobo, G.S.; pathways have a spectrum of clinical effectiveness against viral infections, malignant diseases, Pereira, P.; Freire, M.G.; Neves, M.C.; and multiple sclerosis. -
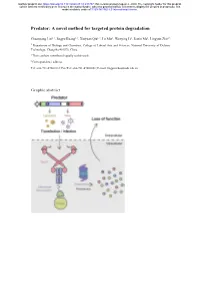
A Novel Method for Targeted Protein Degradation
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Predator: A novel method for targeted protein degradation Chuanyang Liu1, †, Jingyu Kuang1, †, Xinyuan Qiu1, †, Lu Min1, Wenying Li1, Jiaxin Ma1, Lingyun Zhu1,* 1 Department of Biology and Chemistry, College of Liberal Arts and Sciences, National University of Defense Technology, Changsha 410073, China † These authors contributed equally to this work. ∗ Correspondence address. Tel: +86-731-87001812; Fax/Tel: +86-731-87001801; E-mail: [email protected] Graphic abstract bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Protein expression and degradation are fundamental to cell function and physiological status of organisms. Interfering with protein expression not only provides powerful strategies to analyze the function of proteins but also inspires effective treatment methods for diseases caused by protein dysfunction. Recently, harnessing the power of the ubiquitin-proteasome system for targeted protein degradation (TPD) has become the focus of researches. Over the past two decades, TPD technologies, such as E3 ligase modification, PROTACs, and the Trim-Away method, have successfully re-oriented the ubiquitin-proteasome pathway and thus degraded many pathogenic proteins and even "undruggable" targets. -

Peginesatide for Anaemia in Chronic Kidney Disease – First and Second Line
Peginesatide for anaemia in chronic kidney disease – first and second line June 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement on the safety, efficacy or effectiveness of the health technology covered and should not be used for commercial purposes. The NIHR Horizon Scanning Centre Research Programme is part of the National Institute for Health Research www.nhsc-healthhorizons.org.uk June 2012 Peginesatide for anaemia in chronic kidney disease – first and second line Target group • Symptomatic anaemia: patients on dialysis with chronic kidney disease (CKD) – first and second line. Background Human erythropoietin (EPO) is a protein produced by the kidneys that stimulates the production of red blood cells. Because the kidney is the sole source of EPO synthesis in adults, the reduction in kidney mass that occurs in progressive CKD often causes impairment of EPO production, resulting in anaemia. Technology description Peginesatide (AF-37702) is a synthetic peptide mimetic of EPO that binds directly to the erythropoietin receptor on red blood cell precursors to stimulate red cell formation. Peginesatide is intended as a substitute for currently available erythropoiesis stimulating agents (ESAs) in the treatment of anaemia in CKD dialysis patients. It is administered intravenously (IV) or subcutaneously (SC) at 0.04 mg/kg once monthly. After the initial dose, doses may be titrated to achieve a haemoglobin (Hb) level in the range of 10 to 12g/dL. Peginesatide is also in phase II clinical trials for pure red cell aplasia. -

Pipelinetr Ends
June 2011 PIPELINE TRxENDS PIPELINE TR ENDS is produced by the x 1-3 Promising New Agents 5 Industry Trends University of Massachusetts Medical School’s 4 Projected Generic Entry 5 Additional Promising Clinical Pharmacy Services division and 4 Investigational Indications New Agents distributed to our clients twice yearly. 4 FDA Updates In This Issue Promising New Promising New Agents Agents Aflibercept Drug Name: Aclidinium bromide Drug Name: Aflibercept BLA submitted Manufacturer: Almirall, Forest Manufacturer: Regeneron, Bayer for wet age- Phase III Indication: COPD BLA Indication: Wet AMD related macular Formulation: Dry powder inhaler Formulation: Intravitreal injection degeneration Dimethyl fumarate Aclidinium bromide, a long-acting, inhaled Aflibercept is under FDA review for the treatment NDA submission anticholinergic bronchodilator, is an antagonist at of the neovascular form of age-related macular planned for the M2 and M3 muscarinic receptors. It is being degeneration (wet AMD). By inhibiting vascular relapsing-remitting multiple sclerosis studied for the treatment of chronic obstructive endothelial and placental growth factors, aflibercept pulmonary disease (COPD). may reduce the abnormal growth of blood vessels Projected Three Phase III, randomized, double-blind trials that damage the retina through blood and fluid leaks. Generic Entry compared aclidinium bromide 200 μg and 400 μg In two double-blind, non-inferiority, Phase III ® Nasacort AQ twice daily to placebo in patients with moderate to studies, VIEW 1 (N=1,217) and VIEW 2 (N=1,240), Levaquin® severe COPD. In each study, the primary endpoint patients with wet AMD were randomized to Uroxatral® Anzemet® was the change in morning trough forced expiratory intravitreal aflibercept 0.5 mg monthly, 2 mg monthly, Zyprexa® volume in one second (FEV1), from baseline to week or 2 mg every two months following three monthly Zyprexa® Zydis® 12. -

Management of Anemia on Hemodialysis
Chapter 17 Management of Anemia on Hemodialysis Konstantinos Pantelias and Eirini Grapsa Additional information is available at the end of the chapter http://dx.doi.org/10.5772/ 52399 1. Introduction The definition of anemia is controversial. The WHO defines anemia as hemoglobin (Hb)<13 g/dL for men and <12 g/dL for women [1]. The National Kidney Foundation's Kidney Dis‐ ease Outcomes Quality Initiative, which is the criteria used for Medicare reimbursement, de‐ fines anemia in adult men and postmenopausal women as Hb<12 g/dL, or <11 g/dL in a premenopausal woman [2]. Anemia represents a significant problem to deal with in patients with chronic kidney disease (CKD) on hemodialysis (HD). Renal anemia is typically an iso‐ lated normochromic, normocytic anemia with no leukopenia or thrombocytopenia [3]. This is a frequent complication and contributes considerably to reduced quality of life (QoL) [4-6] of patients with CKD. It has also been associated with a number of adverse clinical out‐ comes, increased morbidity and mortality [5, 7-13]. In general, there is a progressive increase in the incidence and severity of anemia with declining renal function. The reported preva‐ lence of anemia by CKD stage varies significantly and depends, to a large extent, on the def‐ inition of anemia and whether study participants selected from the general population, are at a high risk for CKD. Data from the National Health and Nutrition Examination Survey (NHANES) showed that the distribution of Hb levels starts to fall at an estimated glomeru‐ lar filtration rate (eGFR) of less than 75 ml/min per 1.73 m2 in men and 45 ml/min per 1.73 m2 in women [14]. -

WHO Drug Information Vol
WHO Drug Information Vol. 26, No. 4, 2012 WHO Drug Information Contents International Regulatory Regulatory Action and News Harmonization New task force for antibacterial International Conference of Drug drug development 383 Regulatory Authorities 339 NIBSC: new MHRA centre 383 Quality of medicines in a globalized New Pakistan drug regulatory world: focus on active pharma- authority 384 ceutical ingredients. Pre-ICDRA EU clinical trial regulation: public meeting 352 consultation 384 Pegloticase approved for chronic tophaceous gout 385 WHO Programme on Tofacitinib: approved for rheumatoid International Drug Monitoring arthritis 385 Global challenges in medicines Rivaroxaban: extended indication safety 362 approved for blood clotting 385 Omacetaxine mepesuccinate: Safety and Efficacy Issues approved for chronic myelo- Dalfampridine: risk of seizure 371 genous leukaemia 386 Sildenafil: not for pulmonary hyper- Perampanel: approved for partial tension in children 371 onset seizures 386 Interaction: proton pump inhibitors Regorafenib: approved for colorectal and methotrexate 371 cancer 386 Fingolimod: cardiovascular Teriflunomide: approved for multiple monitoring 372 sclerosis 387 Pramipexole: risk of heart failure 372 Ocriplasmin: approved for vitreo- Lyme disease test kits: limitations 373 macular adhesion 387 Anti-androgens: hepatotoxicity 374 Florbetapir 18F: approved for Agomelatine: hepatotoxicity and neuritic plaque density imaging 387 liver failure 375 Insulin degludec: approved for Hypotonic saline in children: fatal diabetes mellitus