Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Induction of a TRAIL Mediated Suicide Program by Interferon Alpha in Primary E€Usion Lymphoma
Oncogene (2001) 20, 7029 ± 7040 ã 2001 Nature Publishing Group All rights reserved 0950 ± 9232/01 $15.00 www.nature.com/onc Induction of a TRAIL mediated suicide program by interferon alpha in primary eusion lymphoma Ngoc L Toomey1,4, Vadim V Deyev2,4, Charles Wood3, Lawrence H Boise2, Duncan Scott1, Lei Hua Liu1, Lisa Cabral1, Eckhard R Podack2, Glen N Barber2 and William J Harrington Jr*,1 1Department of Medicine University of Miami School of Medicine, Miami, Florida, FL 33136, USA; 2Department of Microbiology and Immunology, University of Miami School of Medicine, Miami, Florida, FL 33136, USA; 3School of Biological Sciences, University of Nebraska, Lincoln, Nebraska, NE 68588, USA Gammaherpes viruses are often detected in lymphomas Virus (EBV) or Human Herpes Virus Type 8 (HHV-8) arising in immunocompromised patients. We have found have been isolated from lymphomas found in im- that Azidothymidine (AZT) alone induces apoptosis in munosuppressed organ transplant recipients, children Epstein Barr Virus (EBV) positive Burkitt's lymphoma with hereditary immunode®ciencies and patients with (BL) cells but requires interferon alpha (IFN-a) to induce acquired immunode®ciency (AIDS) (Swinnen, 1999; apoptosis in Human Herpes Virus Type 8 (HHV-8) Goldsby and Carroll, 1998; Knowles, 1999). Many of positive Primary Eusion Lymphomas (PEL). Our these tumors can be categorized into distinct subtypes analysis of a series of AIDS lymphomas revealed that based on a variety of morphologic and molecular IFN-a selectively induced very high levels of the Death criteria. For example, AIDS associated large cell Receptor (DR) tumor necrosis factor-related apoptosis- diuse or immunoblastic lymphomas (DLCL, IBL) inducing ligand (TRAIL) in HHV-8 positive PEL lines are often EBV positive while AIDS associated Burkitt's and primary tumor cells whereas little or no induction lymphomas (BL) less frequently contain EBV (Gaidano was observed in primary EBV+ AIDS lymphomas and et al., 1994). -

Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection Against Classical Swine Fever Virus
Article Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus Yusmel Sordo-Puga 1, Marisela Suárez-Pedroso 1 , Paula Naranjo-Valdéz 2, Danny Pérez-Pérez 1, Elaine Santana-Rodríguez 1, Talia Sardinas-Gonzalez 1, Mary Karla Mendez-Orta 1, Carlos A. Duarte-Cano 1, Mario Pablo Estrada-Garcia 1 and María Pilar Rodríguez-Moltó 1,* 1 Animal Biotechnology Department, Center for Genetic Engineering and Biotechnology, P.O. Box 6162, Havana 10600, Cuba; [email protected] (Y.S.-P.); [email protected] (M.S.-P.); [email protected] (D.P.-P.); [email protected] (E.S.-R.); [email protected] (T.S.-G.); [email protected] (M.K.M.O.); [email protected] (C.A.D.); [email protected] (M.P.E.) 2 Central Laboratory Unit for Animal Health (ULCSA), Havana 11400, Cuba; [email protected] * Correspondence: [email protected]; Tel.: +53-7-2504419 Abstract: Live attenuated C-strain classical swine fever vaccines provide early onset protection. These vaccines confer effective protection against the disease at 5–7 days post-vaccination. It was previously reported that intramuscular administration of the Porvac® vaccine protects against highly virulent Citation: Sordo-Puga, Y.; classical swine fever virus (CSFV) “Margarita” strain as early as seven days post-vaccination. In Suárez-Pedroso, M.; Naranjo-Valdéz, order to identify how rapidly protection against CSFV is conferred after a single dose of the Porvac® P.; Pérez-Pérez, D.; subunit vaccine E2-CD154, 15 swine, vaccinated with a single dose of Porvac®, were challenged Santana-Rodríguez, E.; 3 intranasally at five, three, and one day post-vaccination with 2 × 10 LD50 of the highly pathogenic Sardinas-Gonzalez, T.; Mendez-Orta, Cuban “Margarita” strain of the classical swine fever virus. -

Current Therapies for Chronic Hepatitis C
Southern Illinois University Edwardsville SPARK Pharmacy Faculty Research, Scholarship, and Creative Activity School of Pharmacy 1-2011 Current Therapies for Chronic Hepatitis C McKenzie C. Ferguson Southern Illinois University Edwardsville, [email protected] Follow this and additional works at: https://spark.siue.edu/pharmacy_fac Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Ferguson, McKenzie C., "Current Therapies for Chronic Hepatitis C" (2011). Pharmacy Faculty Research, Scholarship, and Creative Activity. 4. https://spark.siue.edu/pharmacy_fac/4 This Article is brought to you for free and open access by the School of Pharmacy at SPARK. It has been accepted for inclusion in Pharmacy Faculty Research, Scholarship, and Creative Activity by an authorized administrator of SPARK. For more information, please contact [email protected],[email protected]. Chronic Hepatitis C & Current Therapies 1 Review of Chronic Hepatitis C & Current Therapies Reviews of Therapeutics Pharmacotherapy McKenzie C. Ferguson, Pharm.D., BCPS From the School of Pharmacy, Southern Illinois University Edwardsville, Department of Pharmacy Practice, Edwardsville, Illinois. Address for reprint requests : Southern Illinois University Edwardsville School of Pharmacy 220 University Park Drive, Ste 1037 Edwardsville, IL 62026-2000 Email: [email protected] Keywords : hepatitis C, HCV, ribavirin, interferon, peginterferon alfa-2a, peginterferon alfa-2b, albinterferon, taribavirin, telaprevir, therapeutic efficacy, safety The author received no sources of support in the form of grants, equipment, or drugs. Chronic Hepatitis C & Current Therapies 2 ABSTRACT Hepatitis C virus affects more than 180 million people worldwide and as many as 4 million people in the United States. Given that most patients are asymptomatic until late in disease progression, diagnostic screening and evaluation of patients that display high-risk behaviors associated with acquisition of hepatitis C should be performed. -

Age- and Gender-Specific Modulation of Serum Osteopontin and Interferon-Α by Osteopontin Genotype in Systemic Lupus Er
Genes and Immunity (2009) 10, 487–494 & 2009 Macmillan Publishers Limited All rights reserved 1466-4879/09 $32.00 www.nature.com/gene ORIGINAL ARTICLE Age- and gender-specific modulation of serum osteopontin and interferon-a by osteopontin genotype in systemic lupus erythematosus SN Kariuki1, JG Moore1, KA Kirou2,MKCrow2, TO Utset1 and TB Niewold1 1Section of Rheumatology, University of Chicago, Chicago, IL, USA and 2Mary Kirkland Center for Lupus Research, Hospital for Special Surgery, New York, NY, USA Osteopontin (OPN) is a multifunctional cytokine involved in long bone remodeling and immune system signaling. Additionally, OPN is critical for interferon-a (IFN-a) production in murine plasmacytoid dendritic cells. We have previously shown that IFN-a is a heritable risk factor for systemic lupus erythematosus (SLE). Genetic variants of OPN have been associated with SLE susceptibility, and one study suggests that this association is particular to men. In this study, the 3 0 UTR SLE-risk variant of OPN (rs9138C) was associated with higher serum OPN and IFN-a in men (P ¼ 0.0062 and P ¼ 0.0087, respectively). In women, the association between rs9138 C and higher serum OPN and IFN-a was restricted to younger subjects, and risk allele carriers showed a strong age-related genetic effect of rs9138 genotype on both serum OPN and IFN-a (Po0.0001). In African- American subjects, the 5 0 region single nucleotide polymorphisms, rs11730582 and rs28357094, were associated with anti- RNP antibodies (odds ratio (OR) ¼ 2.9, P ¼ 0.0038 and OR ¼ 3.9, P ¼ 0.021, respectively). Thus, we demonstrate two distinct genetic influences of OPN on serum protein traits in SLE patients, which correspond to previously reported SLE-risk variants. -

Interferon-Γ Enhances Interleukin 12 Production in Rheumatoid Synovial
Interferon-γ Enhances Interleukin 12 Production in Rheumatoid Synovial Cells via CD40-CD154 Dependent and Independent Pathways MINETAKE KITAGAWA, HIROSHI SUZUKI, YOSHIHIRO ADACHI, HIROSHI NAKAMURA, SHINICHI YOSHINO, and TAKAYUKI SUMIDA ABSTRACT. Objective. To determine the role of interferon-γ (IFN-γ) in CD40-CD154 dependent production of interleukin 12 (IL-12) by synovial cells of patients with rheumatoid arthritis (RA). Methods. We examined the effects of IFN-γ, tumor necrosis factor-α (TNF-α), and granulocyte- macrophage colony stimulating factor (GM-CSF) on CD40 expression on CD68+ synovial macrophage-lineage cells (SMC). The effects of IFN-γ and soluble CD154 (sCD154) on IL-12 production by RA synovial cells were determined by ELISA. Results. CD68+ SMC expressed substantial levels of CD40. IFN-γ, but not TNF-α or GM-CSF, markedly upregulated CD40 expression on CD68+ SMC. IFN-γ also dose dependently increased IL- γ 12 production by synovial cells. The effects of IFN- on CD40 expression (EC50 = 127.4 U/ml) were observed at a concentration 19 times lower than the effects on IL-12 production (EC50 = 6.8 U/ml). Treatment with IFN-γ at a concentration low enough to augment CD40 expression but not IL-12 production enhanced spontaneous IL-12 production synergy with sCD154. The synergistic enhance- ment of spontaneous IL-12 production was abrogated by CD40-Fc. In contrast, IL-12 production induced by high concentration of IFN-γ was not neutralized by CD40-Fc. Conclusion. IFN-γ enhanced IL-12 production via both CD40-CD154 dependent and independent pathways in RA synovium. IFN-γ may play a crucial role in the development of RA synovitis through regulation of IL-12 production. -

Dysregulated Interferon Response Underlying Severe COVID-19
viruses Review Dysregulated Interferon Response Underlying Severe COVID-19 LeAnn Lopez y, Peter C. Sang y, Yun Tian y and Yongming Sang * Department of Agricultural and Environmental Sciences, College of Agriculture, Tennessee State University, 3500 John A. Merritt Boulevard, Nashville, TN 37209, USA; [email protected] (L.L.); [email protected] (P.C.S.); [email protected] (Y.T.) * Correspondence: [email protected]; Tel.: +1-615-963-5183 These authors contributed equally. y Academic Editor: Andrew Davidson Received: 27 October 2020; Accepted: 9 December 2020; Published: 13 December 2020 Abstract: Innate immune interferons (IFNs), including type I and III IFNs, constitute critical antiviral mechanisms. Recent studies reveal that IFN dysregulation is key to determine COVID-19 pathogenesis. Effective IFN stimulation or prophylactic administration of IFNs at the early stage prior to severe COVID-19 may elicit an autonomous antiviral state, restrict the virus infection, and prevent COVID-19 progression. Inborn genetic flaws and autoreactive antibodies that block IFN response have been significantly associated with about 14% of patients with life-threatening COVID-19 pneumonia. In most severe COVID-19 patients without genetic errors in IFN-relevant gene loci, IFN dysregulation is progressively worsened and associated with the situation of pro-inflammation and immunopathy, which is prone to autoimmunity. In addition, the high correlation of severe COVID-19 with seniority, males, and individuals with pre-existing comorbidities will be plausibly explained by the coincidence of IFN aberrance in these situations. Collectively, current studies call for a better understanding of the IFN response regarding the spatiotemporal determination and subtype-specificity against SARS-CoV-2 infections, which are warranted to devise IFN-related prophylactics and therapies. -

Elimination Pathways of Fusion Protein and Peptide Drugs
Review Volume 11, Issue 2, 2021, 9139 - 9147 https://doi.org/10.33263/BRIAC112.91399147 Elimination Pathways of Fusion Protein and Peptide Drugs Ali Khodadoust 1 , Hossein Aghamollaei 2 , Ali Mohammad Latifi 1 , Kazem Hasanpour 3 , Mahdi Kamali 4 , Hamid Tebyanian 5 , Gholamreza Farnoosh 1,* 1 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 2 Chemical Injuries Research Center, Systems biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran 3 Sabzevar University of Medical Sciences, School of Medicine, Sabzevar, Iran 4 Nanobiotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran 5 Research Center for Prevention of Oral and Dental Diseases, Baqiyatallah University of Medical Sciences, Tehran, Iran * Correspondence: [email protected]; Scopus Author ID 55855454400 Received: 28.07.2020; Revised: 25.08.2020; Accepted: 27.08.2020; Published: 1.09.2020 Abstract: Fusion proteins have been known as an interesting subject for scientific researches in improving properties or making a new function by synergistically incorporating two protein domains into one complex. Fusion proteins are created by ligation of two or more protein domains in a single polypeptide with functional properties. Improvement of therapeutic function is one of the main goals for developing these products. Elimination from the body is one of the most important points that should be considered in the design and production of fusion proteins. This review describes some of the most important excretion/elimination pathways of fusion peptide and protein drugs as well as serious elimination challenges in the development and manufacturing of fusion proteins. Keywords: Fusion proteins; Therapy; Pharmacokinetics; Serum half-life; Peptide. -

Pegasys, INN-Peginterferon Alfa-2A
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion and scientific discussion on procedures, which have been finalised before 1 April 2005. For scientific information on procedures after this date please refer to module 8B. 1. Introduction Peginterferon alfa-2a is a polyethylene glycol (PEG)-modified form of human recombinant interferon alfa-2a intended for the treatment of adult patients with chronic hepatitis C (CHC) or chronic hepatitis B (CHB). Chronic hepatitis C is a major public health problem: hepatitis C virus (HCV) is responsible for a large proportion of chronic liver disease, accounting for 70% of cases of chronic hepatitis in industrialised countries. Globally there are an estimated 150 million chronic carriers of the virus, including 5 million in Western Europe. Without treatment approximately 30% of those infected with HCV will develop cirrhosis over a time frame of 30 years or more. For those with HCV-related cirrhosis, the prognosis is poor – a significant proportion will develop a life-threatening complication (either decompensated liver disease or an hepatocellular carcinoma) within a few years. The only therapy for those with advanced cirrhosis is liver transplantation, which carries a high mortality. In those who survive transplantation, viral recurrence in the new liver is almost inevitable and a significant proportion of infected liver grafts develop a progressive fibrosis that leads to recurrence of cirrhosis within 5 years. Interferon alfa monotherapy has been shown to be effective for the treatment of chronic hepatitis although sustained response rates occurred in approximately 15 to 30 % of patients treated for long duration (12-18 months). The current reference therapy is interferon alpha in combination with ribavirin, which resulted in an increase in biochemical and virological sustained response rates to approximately 40 % in naïve patients. -

Antigen-Specific Induced Foxp3 Regulatory T Cells Are Generated
Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade Ivana R. Ferrer, Maylene E. Wagener, Minqing Song, Allan D. Kirk, Christian P. Larsen, and Mandy L. Ford1 Emory Transplant Center and Department of Surgery, Emory University, Atlanta, GA 30322 Edited by James P. Allison, Memorial Sloan-Kettering Cancer Center, New York, NY, and approved November 4, 2011 (received for review April 7, 2011) Blockade of the CD40/CD154 pathway potently attenuates T-cell in vivo accumulation of Treg and deletion of graft-specific ef- responses in models of autoimmunity, inflammation, and trans- fectors after CD40/CD154 blockade has not been investigated. plantation. Indeed, CD40 pathway blockade remains one of the To address these questions, we used an ovalbumin (OVA)- most powerful methods of prolonging graft survival in models of expressing transgenic mouse model (20), which allowed us to di- + + transplantation. But despite this effectiveness, the cellular and rectly track both donor-reactive CD4 and CD8 T-cell responses molecular mechanisms underlying the protective effects of CD40 simultaneously over time. Although previous studies have used pathway blockade are incompletely understood. Furthermore, the transgenic models to assess the degree of T-cell deletion after CD154/CD40 blockade (16, 21), none has systematically inter- relative contributions of deletion, anergy, and regulation have not + been measured in a model in which donor-reactive CD4+ and CD8+ rogated the impact of anti-CD154 on the kinetics of both CD4 and CD8+ T-cell deletion and acquisition of effector function. T-cell responses can be assessed simultaneously. To investigate the fi Our results indicated that although CD40/CD154 blockade alone impact of CD40/CD154 pathway blockade on graft-speci c T-cell delayed donor-reactive CD4+ and CD8+ T-cell expansion and responses, a transgenic mouse model was used in which recipients differentiation, the addition of DST was required for antigen- fi + + containing ovalbumin-speci cCD4 and CD8 TCR transgenic T specific T-cell deletion. -

CUE-101, a Novel Fc Fusion Protein for Selective Targeting and Expansion of Anti-Tumor T Cells for Treatment of HPV-Driven Malignancies
CUE-101, a novel Fc fusion protein for selective targeting and expansion of anti-tumor T cells for treatment of HPV-driven malignancies Natasha Girgis1, Steven N. Quayle1, Alyssa Nelson1, Dharma Raj Thapa1, Sandrine Hulot1, Lauren Kraemer1, Miguel Moreta1, Zohra Merazga1, Robert Ruidera1, Dominic Beal1, Mark Haydock1, Jonathan Soriano1, Luke Witt1, Jessica Ryabin1, Emily Spaulding1, John F. Ross1, Saso Cemerski1, Anish Suri1, Rodolfo Chaparro1, Ronald Seidel1, Kenneth J. Pienta2, Mary C. Simcox1 1Cue Biopharma, Cambridge, Massachusetts; 2The James Buchanan Brady Urological Institute and the Department of Urology, Johns Hopkins School of Medicine, Baltimore, Maryland + + Background CUE-101 selectively expands E711-20-specific CD8 T mCUE-101 expands functional antigen-specific CD8 T • Human papilloma virus (HPV) is responsible for 72% of oropharyngeal, 90% of cervical, 90% cells from healthy human PBMCs cells in the tumor and the periphery of anal, and 71% of vulvar, vaginal, or penile cancers, causing significant morbidity and A. B. A. B. C. 9 mortality worldwide. Innovative therapies are urgently needed for these malignancies, Vehicle 100 nM CUE-101 107 15 Peripheral Blood particularly in the largely incurable metastatic setting. 106 10 5 • The E7 oncoprotein is constitutively expressed in HPV-associated cancers, is necessary for 105 ⍺PD-1 5 PE 4 Vehicle mCUE-101 Combo - 10 initiation and maintenance of malignant transformation, and is genetically conserved in 1 cancer (Mirabello 2017). 103 1 1 102 E7-Specific of CD8+ T cells % IFNg+,TNFa+ -

Interferon Gamma Release Assay (IGRA) Interferon Gamma Release Assay (IGRA) Is a Blood Test That Has Recently Been Introduced As Another Method to Diagnose LTBI
Section 4: TB Screening These cases also included patients that did not have previous exposure to tuberculin. It is imperative that health care providers should have Epinephrine Hypochloride solution (1:1000) and other appropriate agents available for immediate use in the case of a hypersensitivity reaction. As per NACI, recommendations all patients must be monitored for 15 minutes after inoculation. Interferon Gamma Release Assay (IGRA) Interferon gamma release assay (IGRA) is a blood test that has recently been introduced as another method to diagnose LTBI. Therefore, it can complement the TST in certain situations. In general, IGRAs are more specific than the TST in populations vaccinated with BCG, especially if BCG is given after infancy or multiple times. When a person is exposed to MTB it produces many immune cells, which produce various proteins. Among these proteins are interferon gamma or IFN-y. The interferon gamma release assay (IGRA) is a blood test that measures IFN-y. Therefore, if a person has been exposed and infected with TB, IFN-y can be detected with this test. There are two IGRA tests used in Canada, the T-SPOT.TB test and the QuantiFERON®-TB Gold test. Provincial Laboratory (ProvLab) Alberta uses QuantiFERON®-TB Gold (QFT) test in the NWT. Specimens must be received by the regional laboratory and incubated for 16–24 hours. This limits availability of this test in the NWT. IGRA can only be ordered through the Office of the Chief Public Health Officer and Stanton Regional Hospital Specialists who are involved in TB management and treatment. IGRAs require laboratories with adequate equipment and trained personnel to perform the assays. -
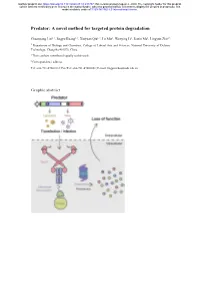
A Novel Method for Targeted Protein Degradation
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Predator: A novel method for targeted protein degradation Chuanyang Liu1, †, Jingyu Kuang1, †, Xinyuan Qiu1, †, Lu Min1, Wenying Li1, Jiaxin Ma1, Lingyun Zhu1,* 1 Department of Biology and Chemistry, College of Liberal Arts and Sciences, National University of Defense Technology, Changsha 410073, China † These authors contributed equally to this work. ∗ Correspondence address. Tel: +86-731-87001812; Fax/Tel: +86-731-87001801; E-mail: [email protected] Graphic abstract bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.231787; this version posted August 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Protein expression and degradation are fundamental to cell function and physiological status of organisms. Interfering with protein expression not only provides powerful strategies to analyze the function of proteins but also inspires effective treatment methods for diseases caused by protein dysfunction. Recently, harnessing the power of the ubiquitin-proteasome system for targeted protein degradation (TPD) has become the focus of researches. Over the past two decades, TPD technologies, such as E3 ligase modification, PROTACs, and the Trim-Away method, have successfully re-oriented the ubiquitin-proteasome pathway and thus degraded many pathogenic proteins and even "undruggable" targets.