Monogenea, Microcotylidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -
HELCOM Red List
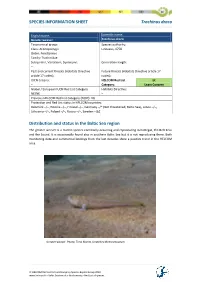
SPECIES INFORMATION SHEET Trachinus draco English name: Scientific name: Greater weever Trachinus draco Taxonomical group: Species authority: Class: Actinopterygii Linnaeus, 1758 Order: Perciformes Family: Trachinidae Subspecies, Variations, Synonyms: Generation length: – Past and current threats (Habitats Directive Future threats (Habitats Directive article 17 article 17 codes): – codes): – IUCN Criteria: HELCOM Red List LC – Category: Least Concern Global / European IUCN Red List Category Habitats Directive: NE/NE – Previous HELCOM Red List Category (2007): VU Protection and Red List status in HELCOM countries: Denmark –/–, Estonia –/–, Finland –/–, Germany –/* (Not threatened, Baltic Sea), Latvia –/–, Lithuania –/–, Poland –/–, Russia –/–, Sweden –/LC Distribution and status in the Baltic Sea region The greater weever is a marine species commonly occurring and reproducing in Kattegat, the Belt Seas and the Sound. It is occasionally found also in southern Baltic Sea but it is not reproducing there. Both monitoring data and commercial landings from the last decades show a positive trend in the HELCOM area. Greater weaver. Photo: Timo Moritz, Deutches Meeresmuseum. © HELCOM Red List Fish and Lamprey Species Expert Group 2013 www.helcom.fi > Baltic Sea trends > Biodiversity > Red List of species SPECIES INFORMATION SHEET Trachinus draco Fig.1 Catch per unit effort (number per trawling hour) of greater weever in international bottom trawl surveys in Kattegat (IBTS) during third quarter of the year (Linear fit and correlation coefficient -

Download E-Book (PDF)
African Journal of Biotechnology Volume 14 Number 33, 19 August, 2015 ISSN 1684-5315 ABOUT AJB The African Journal of Biotechnology (AJB) (ISSN 1684-5315) is published weekly (one volume per year) by Academic Journals. African Journal of Biotechnology (AJB), a new broad-based journal, is an open access journal that was founded on two key tenets: To publish the most exciting research in all areas of applied biochemistry, industrial microbiology, molecular biology, genomics and proteomics, food and agricultural technologies, and metabolic engineering. Secondly, to provide the most rapid turn-around time possible for reviewing and publishing, and to disseminate the articles freely for teaching and reference purposes. All articles published in AJB are peer- reviewed. Submission of Manuscript Please read the Instructions for Authors before submitting your manuscript. The manuscript files should be given the last name of the first author Click here to Submit manuscripts online If you have any difficulty using the online submission system, kindly submit via this email [email protected]. With questions or concerns, please contact the Editorial Office at [email protected]. Editor-In-Chief Associate Editors George Nkem Ude, Ph.D Prof. Dr. AE Aboulata Plant Breeder & Molecular Biologist Plant Path. Res. Inst., ARC, POBox 12619, Giza, Egypt Department of Natural Sciences 30 D, El-Karama St., Alf Maskan, P.O. Box 1567, Crawford Building, Rm 003A Ain Shams, Cairo, Bowie State University Egypt 14000 Jericho Park Road Bowie, MD 20715, USA Dr. S.K Das Department of Applied Chemistry and Biotechnology, University of Fukui, Japan Editor Prof. Okoh, A. I. N. -

Download the Abstract Book
1 Exploring the male-induced female reproduction of Schistosoma mansoni in a novel medium Jipeng Wang1, Rui Chen1, James Collins1 1) UT Southwestern Medical Center. Schistosomiasis is a neglected tropical disease caused by schistosome parasites that infect over 200 million people. The prodigious egg output of these parasites is the sole driver of pathology due to infection. Female schistosomes rely on continuous pairing with male worms to fuel the maturation of their reproductive organs, yet our understanding of their sexual reproduction is limited because egg production is not sustained for more than a few days in vitro. Here, we explore the process of male-stimulated female maturation in our newly developed ABC169 medium and demonstrate that physical contact with a male worm, and not insemination, is sufficient to induce female development and the production of viable parthenogenetic haploid embryos. By performing an RNAi screen for genes whose expression was enriched in the female reproductive organs, we identify a single nuclear hormone receptor that is required for differentiation and maturation of germ line stem cells in female gonad. Furthermore, we screen genes in non-reproductive tissues that maybe involved in mediating cell signaling during the male-female interplay and identify a transcription factor gli1 whose knockdown prevents male worms from inducing the female sexual maturation while having no effect on male:female pairing. Using RNA-seq, we characterize the gene expression changes of male worms after gli1 knockdown as well as the female transcriptomic changes after pairing with gli1-knockdown males. We are currently exploring the downstream genes of this transcription factor that may mediate the male stimulus associated with pairing. -

Bouguerche Et Al
Redescription and molecular characterisation of Allogastrocotyle bivaginalis Nasir & Fuentes Zambrano, 1983 (Monogenea: Gastrocotylidae) from Trachurus picturatus (Bowdich) (Perciformes: Carangidae) off the Algerian coast, Mediterranean Sea Chahinez Bouguerche, Fadila Tazerouti, Delphine Gey, Jean-Lou Justine To cite this version: Chahinez Bouguerche, Fadila Tazerouti, Delphine Gey, Jean-Lou Justine. Redescription and molecular characterisation of Allogastrocotyle bivaginalis Nasir & Fuentes Zambrano, 1983 (Monogenea: Gas- trocotylidae) from Trachurus picturatus (Bowdich) (Perciformes: Carangidae) off the Algerian coast, Mediterranean Sea. Systematic Parasitology, Springer Verlag (Germany), 2019, 96 (8), pp.681-694. 10.1007/s11230-019-09883-7. hal-02557974 HAL Id: hal-02557974 https://hal.archives-ouvertes.fr/hal-02557974 Submitted on 29 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bouguerche et al. Allogastrocotyle bivaginalis 1 Systematic Parasitology (2019) 96:681–694 DOI: 10.1007/s11230-019-09883-7 Redescription and molecular characterisation -

Revisión Taxonómica De La Ictiología Marina De Galicia: Clase Actinopteri (Orden Trachiniformes Al Orden Tetraodontiformes)
Nova Acta Científica Compostelana (Bioloxía), 28: 77-104 (2021) - ISSN 2340-0021 ARTÍCULO DE INVESTIGACIÓN Revisión taxonómica de la ictiología marina de Galicia: Clase Actinopteri (Orden Trachiniformes al Orden Tetraodontiformes) Taxonomic review of Galician marine ichthyology: Classe Actinopteri (Order Trachiniformes to Order Tetraodontiformes) *RAFAEL BAÑÓN, TOÑO MAÑO Grupo de Estudos do Medio Mariño (GEMM), Puerto Deportivo s/n 15960 Ribeira, A Coruña, España. *[email protected]; [email protected] (Recibido 29/11/2020; Aceptado 26/03/2021) Resumen En este trabajo se realiza una revisión taxonómica de los peces óseos de Galicia (Clase Actinopteri) del Orden Trachiniformes al Orden Tetraodontiformes, a través de los distintos tratados y publicaciones ictio- lógicas publicadas a lo largo de la historia. Se listan un total de 188 especies, de las cuales 5 se consideran dudosas, al no estar su presencia suficientemente demostrada. Una revisión de la bibliografía y nomenclatura científica nos ha permitido citar nuevas especies para Galicia y reasignar antiguas denominaciones a nuevas especies, subsanando errores de identificación de otros autores. El orden Perciformes, con 145 especies, es el más numeroso de los peces de Galicia. A este orden pertenecen especies de alto interés comercial como el jurel Trachurus trachurus y la caballa Scomber scombrus. El listado incluye también los primeros registros para Galicia de especies de carácter tropical desplazadas hacia el norte debido al cambio climático a lo largo de estas últimas décadas. Algunas de estas especies son el jurelo azul Caranx crysos, el pez globo Lagocephalus laevigatus y el mero tropical Epinephelus aeneus. Palabras clave: Peces óseos, nomenclatura, ictiología, tropicalización. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Towards the Resolution of the Microcotyle Erythrini Species Complex: Description of Microcotyle Isyebi N
Towards the resolution of the Microcotyle erythrini species complex: description of Microcotyle isyebi n. sp. (Monogenea, Microcotylidae) from Boops boops (Teleostei, Sparidae) off the Algerian coast Chahinez Bouguerche, Delphine Gey, Jean-Lou Justine, Fadila Tazerouti To cite this version: Chahinez Bouguerche, Delphine Gey, Jean-Lou Justine, Fadila Tazerouti. Towards the resolution of the Microcotyle erythrini species complex: description of Microcotyle isyebi n. sp. (Monogenea, Microcotylidae) from Boops boops (Teleostei, Sparidae) off the Algerian coast. Parasitology Research, Springer Verlag (Germany), 2019, 118, pp.1417-1428. 10.1007/s00436-019-06293-y. hal-02080519 HAL Id: hal-02080519 https://hal.archives-ouvertes.fr/hal-02080519 Submitted on 29 Apr 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bouguerche et al. Microcotyle isyebi 1 DOI: 10.1007/s00436-019-06293-y ZooBank: urn:lsid:zoobank.org:pub:366371C5-3384-4CE5-A4C2-3AE839EA01DD Towards the resolution of the Microcotyle erythrini species complex: description of Microcotyle isyebi -

Triple Barcoding for a Hyperparasite, Its Parasitic Host, and the Host Itself
Parasite 28, 49 (2021) Ó C. Bouguerche et al., published by EDP Sciences, 2021 https://doi.org/10.1051/parasite/2021044 Available online at: www.parasite-journal.org RESEARCH ARTICLE OPEN ACCESS Triple barcoding for a hyperparasite, its parasitic host, and the host itself: a study of Cyclocotyla bellones (Monogenea) on Ceratothoa parallela (Isopoda) on Boops boops (Teleostei) Chahinez Bouguerche1,4, Fadila Tazerouti1, Delphine Gey2,3, and Jean-Lou Justine4,* 1 Université des Sciences et de la Technologie Houari Boumediene, Faculté des Sciences Biologiques, Laboratoire de Biodiversité et Environnement : Interactions – Génomes, BP 32, El Alia, Bab Ezzouar, Algiers, Algeria 2 Service de Systématique moléculaire, UMS 2700 CNRS, Muséum National d’Histoire Naturelle, Sorbonne Universités, 43 rue Cuvier, CP 26, 75231 Paris Cedex 05, France 3 UMR7245 MCAM, Muséum National d’Histoire Naturelle, 43 rue Cuvier, CP 52, 75231 Paris Cedex 05, France 4 Institut Systématique Évolution Biodiversité (ISYEB), Muséum National d’Histoire Naturelle, CNRS, Sorbonne Université, EPHE, Université des Antilles, 43 rue Cuvier, CP 51, 75231 Paris Cedex 05, France Received 15 October 2020, Accepted 7 May 2021, Published online 7 June 2021 Abstract – Cyclocotyla bellones Otto, 1823 (Diclidophoridae) is a monogenean characterised by an exceptional way of life. It is a hyperparasite that attaches itself to the dorsal face of isopods, themselves parasites in the buccal cavity of fishes. In this study, Cy. bellones was found on Ceratothoa parallela (Otto, 1828), a cymothoid isopod parasite of the sparid fish Boops boops off Algeria in the Mediterranean Sea. We provide, for the first time, molecular barcoding information of a hyperparasitic monogenean, the parasitic crustacean host, and the fish host, with COI sequences. -

Venom Evolution Widespread in Fishes: a Phylogenetic Road Map for the Bioprospecting of Piscine Venoms
Journal of Heredity 2006:97(3):206–217 ª The American Genetic Association. 2006. All rights reserved. doi:10.1093/jhered/esj034 For permissions, please email: [email protected]. Advance Access publication June 1, 2006 Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms WILLIAM LEO SMITH AND WARD C. WHEELER From the Department of Ecology, Evolution, and Environmental Biology, Columbia University, 1200 Amsterdam Avenue, New York, NY 10027 (Leo Smith); Division of Vertebrate Zoology (Ichthyology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Leo Smith); and Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192 (Wheeler). Address correspondence to W. L. Smith at the address above, or e-mail: [email protected]. Abstract Knowledge of evolutionary relationships or phylogeny allows for effective predictions about the unstudied characteristics of species. These include the presence and biological activity of an organism’s venoms. To date, most venom bioprospecting has focused on snakes, resulting in six stroke and cancer treatment drugs that are nearing U.S. Food and Drug Administration review. Fishes, however, with thousands of venoms, represent an untapped resource of natural products. The first step in- volved in the efficient bioprospecting of these compounds is a phylogeny of venomous fishes. Here, we show the results of such an analysis and provide the first explicit suborder-level phylogeny for spiny-rayed fishes. The results, based on ;1.1 million aligned base pairs, suggest that, in contrast to previous estimates of 200 venomous fishes, .1,200 fishes in 12 clades should be presumed venomous. -

APPENDIX 1 Classified List of Fishes Mentioned in the Text, with Scientific and Common Names
APPENDIX 1 Classified list of fishes mentioned in the text, with scientific and common names. ___________________________________________________________ Scientific names and classification are from Nelson (1994). Families are listed in the same order as in Nelson (1994), with species names following in alphabetical order. The common names of British fishes mostly follow Wheeler (1978). Common names of foreign fishes are taken from Froese & Pauly (2002). Species in square brackets are referred to in the text but are not found in British waters. Fishes restricted to fresh water are shown in bold type. Fishes ranging from fresh water through brackish water to the sea are underlined; this category includes diadromous fishes that regularly migrate between marine and freshwater environments, spawning either in the sea (catadromous fishes) or in fresh water (anadromous fishes). Not indicated are marine or freshwater fishes that occasionally venture into brackish water. Superclass Agnatha (jawless fishes) Class Myxini (hagfishes)1 Order Myxiniformes Family Myxinidae Myxine glutinosa, hagfish Class Cephalaspidomorphi (lampreys)1 Order Petromyzontiformes Family Petromyzontidae [Ichthyomyzon bdellium, Ohio lamprey] Lampetra fluviatilis, lampern, river lamprey Lampetra planeri, brook lamprey [Lampetra tridentata, Pacific lamprey] Lethenteron camtschaticum, Arctic lamprey] [Lethenteron zanandreai, Po brook lamprey] Petromyzon marinus, lamprey Superclass Gnathostomata (fishes with jaws) Grade Chondrichthiomorphi Class Chondrichthyes (cartilaginous -

(Muğl ) O Ho Kly Ġġ Ġ Ġcġl
NK NĠ Ġ Ġ N ĠLĠML Ġ N Ġ OK O Ġ G LL K KÖ ĠN (MUĞL ) O HO K L Y Ġġ Ġ ĠCĠLĠĞĠ Y PIL N L K (Dicentrarchus labrax) ÇĠPU (Sparus aurata) LIKL IN ULUN N K OP Ġ L ĠN LĠ L NM Ġ Quyet PHAN VAN U NL Ġ N ĠLĠM LI ANKARA 2020 Her hakkı saklıdır Ö DOKTORA TEZI G LL K KOR Z N (MUĞLA) O SHOR KA SL R Y T ŞT R C L Ğ YAPILAN L VR K (Dicentrarchus labrax) V ÇIPURA (Sparus aurata) ALIKLARIN A ULUNAN KTOPARAZ TL R N L RL NM S Quyet PHAN VAN Ankara niversitesi Fen ilimleri nstit s Su r nleri Ana ilim alı anı man: Prof r Hijran YAVUZCAN u tez alı masında, G ll k Körfezinde ulunan off-shore kafeslerde yeti tiricili i yapılan levrek (Dicentrarchus labrax) ve ipura (Sparus aurata) alıklarında ulunan ektoparazitler mevsimsel olarak incelenmi tir Çalı mada 175 adet levrek ve 175 ipura olmak zere toplam 35 alık incelenmi tir alıklarda yapılan incelemeler soncunda genel parazit prevalansı levrek alıklarında %97 14, ipura alıklarında ise %5 8 olarak elirlenmi tir Levrek alıklarında tespit edilen ektoparazitlerden Diplectanum aequans, Lernanthropus kroyeri ve Amyloodinium ocellatum t rlerinin prevalansı sırasıyla %82.28, %58 85 ve %4 57 olarak elirlenmi tir Levrek alıklarında parazitlere ait ortalama bolluk de erleri; D. aequans i in 4 61± 56, L. kroyeri i in 1 33± 15 ve A. ocellatum i in 0.10± 4 olarak saptanmı tır Levrek alıklarında parazitlerin ortalama yo unlukları, D. aequans i in 5 61± 56, L.