Key Factors Explaining Critical Swimming Speed in Freshwater Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Comparison of the Myxobolus Fauna of Common Barbel from Hungary and Iberian Barbel from Portugal
Vol. 100: 231–248, 2012 DISEASES OF AQUATIC ORGANISMS Published September 12 doi: 10.3354/dao02469 Dis Aquat Org Comparison of the Myxobolus fauna of common barbel from Hungary and Iberian barbel from Portugal K. Molnár1,*, E. Eszterbauer1, Sz. Marton1, Cs. Székely1, J. C. Eiras2 1Institute for Veterinary Medical Research, Centre for Agricultural Research, HAS, POB 18, 1581 Budapest, Hungary 2Departamento de Biologia, e CIIMAR, Faculdade de Ciências, Universidade do Porto, Rua do Campo Alegre, s/n, Edifício FC4, 4169-007 Porto, Portugal ABSTRACT: We compared Myxobolus infection of common barbel Barbus barbus from the Danube River in Hungary with that in Iberian barbel Luciobarbus bocagei from the Este River in Portugal. In Hungary, we recorded 5 known Myxobolus species (M. branchialis, M. caudatus, M. musculi, M. squamae, and M. tauricus) and described M. branchilateralis sp. n. In Portugal we recorded 6 Myxobolus species (M. branchialis, M. branchilateralis sp. n., M. cutanei, M. musculi, M. pfeifferi, and M. tauricus). Species found in the 2 habitats had similar spore morphology and only slight differences were observed in spore shape or measurements. All species showed a spe- cific tissue tropism and had a definite site selection. M. branchialis was recorded from the lamellae of the gills, large plasmodia of M. branchilateralis sp. n. developed at both sides of hemibranchia, M. squamae infected the scales, plasmodia of M. caudatus infected the scales and the fins, and M. tauricus were found in the fins and pin bones. In the muscle, 3 species, M. musculi, M. pfeifferi and M. tauricus were found; however they were found in distinct locations. -

Fish Locomotion: Recent Advances and New Directions
MA07CH22-Lauder ARI 6 November 2014 13:40 Fish Locomotion: Recent Advances and New Directions George V. Lauder Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 02138; email: [email protected] Annu. Rev. Mar. Sci. 2015. 7:521–45 Keywords First published online as a Review in Advance on swimming, kinematics, hydrodynamics, robotics September 19, 2014 The Annual Review of Marine Science is online at Abstract marine.annualreviews.org Access provided by Harvard University on 01/07/15. For personal use only. Research on fish locomotion has expanded greatly in recent years as new This article’s doi: approaches have been brought to bear on a classical field of study. Detailed Annu. Rev. Marine. Sci. 2015.7:521-545. Downloaded from www.annualreviews.org 10.1146/annurev-marine-010814-015614 analyses of patterns of body and fin motion and the effects of these move- Copyright c 2015 by Annual Reviews. ments on water flow patterns have helped scientists understand the causes All rights reserved and effects of hydrodynamic patterns produced by swimming fish. Recent developments include the study of the center-of-mass motion of swimming fish and the use of volumetric imaging systems that allow three-dimensional instantaneous snapshots of wake flow patterns. The large numbers of swim- ming fish in the oceans and the vorticity present in fin and body wakes sup- port the hypothesis that fish contribute significantly to the mixing of ocean waters. New developments in fish robotics have enhanced understanding of the physical principles underlying aquatic propulsion and allowed intriguing biological features, such as the structure of shark skin, to be studied in detail. -

Evaluating 87Sr/86Sr Isotope Ratios and Sr Mass Fractions in Otoliths Of
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.23.453494; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Evaluating 87Sr/86Sr isotope ratios and Sr mass fractions in otoliths of different European freshwater 2 fish species as fishery management tool in an Alpine foreland with limited geological variability 3 Andreas Zitek1,2*, Johannes Oehm3, Michael Schober1, Anastassiya Tchaikovsky1, Johanna Irrgeher5, 4 Anika Retzmann5, Bettina Thalinger4, Michael Traugott3, Thomas Prohaska5 5 1University of Natural Resources and Life Sciences, Vienna, Department of Chemistry, Institute of 6 Analytical Chemistry, Muthgasse 18, 1190 Wien, Austria 7 2FFoQSI GmbH ‐ Austrian Competence Centre for Feed and Food Quality, Safety & Innovation, 8 Technopark 1D, 3430 Tulln, Austria 9 3University of Innsbruck, Department of Zoology, Technikerstraße 25, 6020 Innsbruck, Austria 10 4University of Guelph, 50 Stone Road East, Guelph, N1G2W1, Canada 11 5Department of General, Analytical and Physical Chemistry, Chair of General and Analytical Chemistry, 12 Montanuniversität Leoben, Franz Josef‐Straße 18, 8700 Leoben, Austria 13 14 *Corresponding author: [email protected] 15 16 Highlights 17 Otolith microchemistry applied in in area with limited geological variability 18 Fish transferred, stocked or migrated were identified 19 Regressions between Sr/Ca ratios in water predict Sr mass fractions in otoliths 20 Species specific Sr discrimination from water into otoliths 21 European freshwater fish species assigned to habitat clusters of origin 22 Keywords 23 Strontium isotopes, Sr elemental fingerprint, otolith microchemistry, freshwater fish species, fishery 24 management. -

Copyrighted Material
Index INDEX Note: page numbers in italics refer to fi gures, those in bold refer to tables and boxes. abducens nerve 55 activity cycles 499–522 inhibition 485 absorption effi ciency 72 annual patterns 515, 516, 517–22 interactions 485–6 abyssal zone 393 circadian rhythms 505 prey 445 Acanthaster planci (Crown-of-Thorns Starfi sh) diel patterns 499, 500, 501–2, 503–4, reduction 484 579 504–7 aggressive mimicry 428, 432–3 Acanthocybium (Wahoo) 15 light-induced 499, 500, 501–2, 503–4, aggressive resemblance 425–6 Acanthodii 178, 179 505 aglomerular 52 Acanthomorpha 284–8, 289 lunar patterns 507–9 agnathans Acanthopterygii 291–325 seasonal 509–15 gills 59, 60 Atherinomorpha 293–6 semilunar patterns 507–9 osmoregulation 101, 102 characteristics 291–2 supra-annual patterns 515, 516, 517–22 phylogeny 202 distribution 349, 350 tidal patterns 506–7 ventilation 59, 60 jaws 291 see also migration see also hagfi shes; lampreys Mugilomorpha 292–3, 294 adaptive response 106 agnathous fi shes see jawless fi shes pelagic 405 adaptive zones 534 agonistic interactions 83–4, 485–8 Percomorpha 296–325 adenohypophysis 91, 92 chemically mediated 484 pharyngeal jaws 291 adenosine triphosphate (ATP) 57 sound production 461–2 phylogeny 292, 293, 294 adipose fi n 35 visual 479 spines 449, 450 adrenocorticotropic hormone (ACTH) 92 agricultural chemicals 605 Acanthothoraciformes 177 adrianichthyids 295 air breathing 60, 61–2, 62–4 acanthurids 318–19 adult fi shes 153, 154, 155–7 ammonia production 64, 100–1 Acanthuroidei 12, 318–19 death 156–7 amphibious 60 Acanthurus bahianus -

Habitat Use by Pseudochondrostoma Duriense and Squalius Carolitertii Downstream of a Small-Scale Hydropower Plant
water Article Habitat Use by Pseudochondrostoma duriense and Squalius carolitertii Downstream of a Small-Scale Hydropower Plant Isabel Boavida 1,* , Filipa Ambrósio 2, Maria João Costa 1 , Ana Quaresma 1 , Maria Manuela Portela 1, António Pinheiro 1 and Francisco Godinho 3 1 CERIS, Civil Engineering Research and Innovation for Sustainability, Instituto Superior Técnico, University of Lisbon, 1049-001 Lisbon, Portugal; [email protected] (M.J.C.); [email protected] (A.Q.); [email protected] (M.M.P.); [email protected] (A.P.) 2 Instituto Superior Técnico, University of Lisbon, 1049-001 Lisbon, Portugal; fi[email protected] 3 Hidroerg, Projectos Energéticos, Lda, 1300-365 Lisbon, Portugal; [email protected] * Correspondence: [email protected] Received: 24 July 2020; Accepted: 5 September 2020; Published: 9 September 2020 Abstract: Downstream of small-scale hydropower plants (SHPs), the intensity, frequency and persistence of hydropeaking events often cause an intolerable stress on fish of all life stages. Rapid increases in flow velocity result in fish avoiding unstable habitats and seeking refuge to reduce energy expenditure. To understand fish movements and the habitat use of native Iberian cyprinids in a high-gradient peaking river, 77 individuals were PIT tagged downstream of Bragado SHP in the North of Portugal. Tagged fish species included Pseudochondrostoma duriense and Squalius carolitertii. Fish positions were recorded manually on two different occasions: during hydropeaking events (HP) and without hydropeaking events (NHP). From the 77 tagged fish, we were able to record habitat use for 33 individuals (20 P. duriense and 13 S. -
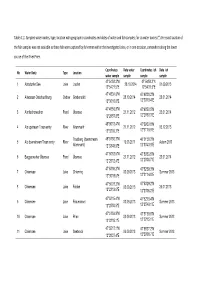
Sampled Water Bodies, Type, Location with Geographic
Table A.1: Sampled water bodies, type, location with geographic coordinates and dates of water and fish samples; for six water bodies (*) the exact location of the fish samples was not available as these fish were captured by fishermen within the investigated lakes, or in one occasion, somewhere along the lower course of the River Prien. Coordinates Date water Coordinates fish Date fish No Water Body Type Location water sample sample sample sample 47°54'52.3"N 47°54'52.3"N 1 Abtsdorfer See Lake Laufen 28.10.2014 01.09.2013 12°54'21.5"E 12°54'21.5"E 47°48'34.6"N 47°48'30.2"N 2 Altwasser Osterbuchberg Oxbow Grabenstätt 28.10.2014 23.01.2014 12°30'10.6"E 12°30'19.4"E 47°48'56.8"N 47°48'53.5"N 3 Almfischerweiher Pond Übersee 21.11.2012 23.01.2014 12°29'57.8"E 12°29'53.3"E 48°00'13.4"N 47°59'53.8"N 4 Alz upstream Traun entry River Altenmarkt 21.11.2012 05.12.2013 12°32'00.3"E 12°31'19.9"E Trostberg (downstream 48°01'50.2"N 48°01'50.2"N 5 Alz downstream Traun entry River 10.03.2011 Autum 2013 Altenmarkt) 12°33'43.9"E 12°33'43.9"E 47°50'52.5"N 47°50'53.5"N 6 Baggerweiher Übersee Pond Übersee 21.11.2012 23.01.2014 12°29'12.4"E 12°29'08.7"E 47°53'09.2"N 47°52'59.3"N 7 Chiemsee Lake Chieming 03.09.2013 Summer 2013 12°30'19.5"E 12°31'14.8"E 47°50'28.3"N 47°50'29.2"N 8 Chiemsee Lake Felden 03.09.2013 26.07.2013 12°23'12.6"E 12°23'06.2"E 47°52'15.4"N 47°52'10.4"N 9 Chiemsee Lake Fraueninsel 03.09.2013 Summer 2013 12°25'50.5"E 12°25'49.1"E 47°51'59.8"N 47°51'59.8"N 10 Chiemsee Lake Prien 03.09.2013 Summer 2013 12°22'15.1"E 12°22'15.1"E 47°55'17.1"N -

Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus Idus
REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1822280 REVIEW Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus idus Mehis Rohtlaa,b, Lorenzo Vilizzic, Vladimır Kovacd, David Almeidae, Bernice Brewsterf, J. Robert Brittong, Łukasz Głowackic, Michael J. Godardh,i, Ruth Kirkf, Sarah Nienhuisj, Karin H. Olssonh,k, Jan Simonsenl, Michał E. Skora m, Saulius Stakenas_ n, Ali Serhan Tarkanc,o, Nildeniz Topo, Hugo Verreyckenp, Grzegorz ZieRbac, and Gordon H. Coppc,h,q aEstonian Marine Institute, University of Tartu, Tartu, Estonia; bInstitute of Marine Research, Austevoll Research Station, Storebø, Norway; cDepartment of Ecology and Vertebrate Zoology, Faculty of Biology and Environmental Protection, University of Lodz, Łod z, Poland; dDepartment of Ecology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia; eDepartment of Basic Medical Sciences, USP-CEU University, Madrid, Spain; fMolecular Parasitology Laboratory, School of Life Sciences, Pharmacy and Chemistry, Kingston University, Kingston-upon-Thames, Surrey, UK; gDepartment of Life and Environmental Sciences, Bournemouth University, Dorset, UK; hCentre for Environment, Fisheries & Aquaculture Science, Lowestoft, Suffolk, UK; iAECOM, Kitchener, Ontario, Canada; jOntario Ministry of Natural Resources and Forestry, Peterborough, Ontario, Canada; kDepartment of Zoology, Tel Aviv University and Inter-University Institute for Marine Sciences in Eilat, Tel Aviv, -

Barbatula Leoparda (Actinopterygii, Nemacheilidae), a New Endemic Species of Stone Loach of French Catalonia
Scientific paper Barbatula leoparda (Actinopterygii, Nemacheilidae), a new endemic species of stone loach of French Catalonia by Camille GAULIARD (1), Agnès DETTAI (2), Henri PERSAT (1, 3), Philippe KEITH (1) & Gaël P.J. DENYS* (1, 4) Abstract. – This study described a new stone loach species in France, Barbatula leoparda, which is endemic to French Catalonia (Têt and Tech river drainages). Seven specimens were compared to 49 specimens of B. bar- batula (Linnaeus, 1758) and 71 specimens of B. quignardi (Băcescu-Meşter, 1967). This new species is char- acterized by the presence of blotches on the belly and the jugular area in individuals longer than 47 mm SL and by a greater interorbital distance (35.5 to 41.8% of the head length). We brought moreover the sequence of two mitochondrial markers (COI and 12S, respectively 652 and 950 bp) of the holotype, which are well distinct from all other species, for molecular identifications. This discovery is important for conservation. Résumé. – Barbatula leoparda (Actinopterigii, Nemacheilidae), une nouvelle espèce endémique de loche fran- che en Catalogne française. © SFI Submitted: 4 Jun. 2018 Cette étude décrit une nouvelle espèce de loche franche en France, Barbatula leoparda, qui est endémique Accepted: 23 Jan. 2019 Editor: G. Duhamel à la Catalogne française (bassins de la Têt et du Tech). Sept spécimens ont été comparés à 49 spécimens de B. barbatula (Linnaeus, 1758) et 71 spécimens de B. quignardi (Băcescu-Meşter, 1967). Cette nouvelle espèce est caractérisée par la présence de taches sur le ventre et dans la partie jugulaire pour les individus d’une taille supérieure à 47 mm LS et par une plus grande distance inter-orbitaire (35,5 to 41,8% de la longueur de la tête). -

総 説 日本産コイ科魚類に寄生する単生類フタゴムシ Eudiplozoon Nipponicum と 近縁未同定種に関す
生物圏科学 Biosphere Sci. 55:39-56 (2016) 総 説 日本産コイ科魚類に寄生する単生類フタゴムシ Eudiplozoon nipponicum と 近縁未同定種に関する解説[付録:亀谷 了博士の研究業績目録] 長澤和也 * 広島大学大学院生物圏科学研究科 〒 739-8528 広島県東広島市鏡山 1-4-4 要 旨 1891–2016年に日本で出版された報文に基づき,日本のコイ科魚類に寄生する単生類の フタゴムシ Eudiplozoon nipponicum (Goto, 1891) と「Diplozoon sp.」と報告されてきた未同定種に 関する知見を纏めた。フタゴムシに関しては,本種の命名者である五島淸太郎による記載,成虫の 形態,宿主,国内分布,生活史,成熟・産卵の季節変化,宿主サイズと寄生状況との関係,病害性 と対策,水産業との関係などを記述した。また,「Diplozoon sp.」と報告されてきた未同定種の 多くはウグイフタゴムシ Paradilpozoon skrjabini Akhmerov, 1974 に同定できる可能性を示唆し た。亀谷 了博士によるフタゴムシ類の研究業績を付録として示した。 キーワード: ウグイフタゴムシ,亀谷 了,魚類寄生虫,コイ科魚類,単生類,フタゴムシ, Diplozoon sp.,Eudiplozoon nipponicum,Paradilpozoon skrjabini 緒 言 広島大学大学院生物圏科学研究科水圏生物生産学講座に属する水産増殖学研究室では,2006年に広島県 中央部にある黒瀬川水系において淡水魚類の寄生虫研究を始めた。これは,本論文の筆者である長澤が 2005年9月に広島大学に赴任した際,魚類寄生虫研究を行うため,広島大学が位置する東広島市と近隣市町 において採集候補地を調査した結果,黒瀬川水系には環境変化に富む本流や支流のほか多くの溜池が大学の 近くにあり,また魚類が豊富でそれらの採集が容易であったことから,学生らと淡水魚類の寄生虫研究を行 うのに好都合であると判断したからである。これまでに研究成果がいくつか公刊されている(Nagasawa et al., 2007, 2013, 2014;Maneepitaksanti and Nagasawa, 2012;Nagasawa and Obe, 2013)。 この研究過程で,2007年に東広島市西条中央地区を流れる黒瀬川本流でギンブナの鰓にフタゴムシ Eudiplozoon nipponicum (Goto, 1891) の寄生を認め,若干の調査を行った(ただし,2009年に同所のギンブ ナ個体群が原因不明のまま消滅し,現在まで回復していないため,研究は中断している)。そして,この研 究を進めるに当たって,コイ科魚類に寄生するフタゴムシとその近縁属種(以下,フタゴムシ類)について, わが国で出版された報文を入手し,過去の知見に接する機会を得た。筆者が知る限り,わが国でフタゴムシ 類に関する知見を総括したのは亀谷(1976)のみで,それ以来,約40年が経過した。この間に,わが国の フタゴムシ類に関する研究が進んだことに加え,最近,フタゴムシ以外にウグイフタゴムシParadilpozoon skrjabini Akhmerov, 1974が報告された(Shimazu et al., 2015)。こうした状況を踏まえて,本解説では,1891 年に記載されて以来,わが国で長い研究の歴史を有するフタゴムシに関する過去の知見を整理し,解説とし て示すことにした。最近報告されたウグイフタゴムシについては,これからの知見の集積を待って,別の機 -

Rough Fish”: Paradigm Shift in the Conservation of Native Fishes Andrew L
PERSPECTIVE Goodbye to “Rough Fish”: Paradigm Shift in the Conservation of Native Fishes Andrew L. Rypel | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, 1 Shields Ave, Davis, CA 95616 | University of California, Davis, Center for Watershed Sciences, Davis, CA. E-mail: [email protected] Parsa Saffarinia | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Caryn C. Vaughn | University of Oklahoma, Oklahoma Biological Survey and Department of Biology, Norman, OK Larry Nesper | University of Wisconsin–Madison, Department of Anthropology, Madison, WI Katherine O’Reilly | University of Notre Dame, Department of Biological Sciences, Notre Dame, IN Christine A. Parisek | University of California, Davis, Center for Watershed Sciences, Davis, CA | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA | The Nature Conservancy, Science Communications, Boise, ID Peter B. Moyle | University of California, Davis, Center for Watershed Sciences, Davis, CA Nann A. Fangue | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Miranda Bell- Tilcock | University of California, Davis, Center for Watershed Sciences, Davis, CA David Ayers | University of California, Davis, Center for Watershed Sciences, Davis, CA | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Solomon R. David | Nicholls State University, Department of Biological Sciences, Thibodaux, LA While sometimes difficult to admit, perspectives of European and white males have overwhelmingly dominated fisheries science and management in the USA. This dynamic is exemplified by bias against “rough fish”— a pejorative ascribing low- to- zero value for countless native fishes. One product of this bias is that biologists have ironically worked against conservation of diverse fishes for over a century, and these problems persist today. -

THE HYDROBIOTOPIC DIVERSITY of the LAKES of the LOWER PRUT RIVER, REPUBLIC of MOLDOVA Mihaela MUNTEANU (PILA)1, Silvius STANCIU
THE HYDROBIOTOPIC DIVERSITY OF THE LAKES OF THE LOWER PRUT RIVER, REPUBLIC OF MOLDOVA Mihaela MUNTEANU (PILA)1, Silvius STANCIU2 1PhD Student, „Dunărea de Jos” University of Galați, Romania, email: [email protected]. 2 PhD Professor „Dunărea de Jos”University of Galați, Romania, email: [email protected] Abstract This paper proposes the presentation and analysis of the fish fauna and the specific benthic resources of the Prut River and its tributaries: Costeşti-Stânca, Beleu, Manta. An important objective of the paper is to present the differences in hydrobiotop diversity in different riparian sectors. A healthy ecosystem has a rich ichthyofauna due to the diversity of vegetation and fish species capable of withstanding resistance to aggressive external factors and threats. The aquatic biodiversity of the Prut River is a major complex consisting of phytoplankton and zooplankton. To optimize production of fish, an important factor is maintaining the balance quantity and quality of the entire water system. From this point of view, the paper presented also quantitative aspects of zoobenthos, which is different in the analyzed areas, being influenced by the degree of pollution, the hydrological regime or the physico-chemical conditions. Research has revealed significant differences between different areas of the aquatic fauna of the Prut, mainly due to the human factor. The study is a preliminary one, preparing a broader analysis of the impact of the human factor in the aquatic areas of the Republic of Moldova. Identifying and analyzing the factors that have led to the modification of aquatic structure and diversity can serve as arguments for achieving viable measures for the protection and sustainable use of natural aquatic resources at national level.