The Microbial Loop – 25 Years Later
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
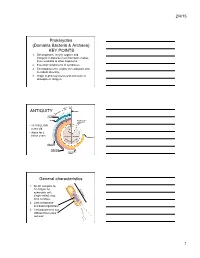
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

Response of Marine Food Webs to Climate-Induced Changes in Temperature and Inflow of Allochthonous Organic Matter
Response of marine food webs to climate-induced changes in temperature and inflow of allochthonous organic matter Rickard Degerman Department of Ecology and Environmental Science 901 87 Umeå Umeå 2015 1 Copyright©Rickard Degerman ISBN: 978-91-7601-266-6 Front cover illustration by Mats Minnhagen Printed by: KBC Service Center, Umeå University Umeå, Sweden 2015 2 Tillägnad Maria, Emma och Isak 3 Table of Contents Abstract 5 List of papers 6 Introduction 7 Aquatic food webs – different pathways Food web efficiency – a measure of ecosystem function Top predators cause cascade effects on lower trophic levels The Baltic Sea – a semi-enclosed sea exposed to multiple stressors Varying food web structures Climate-induced changes in the marine ecosystem Food web responses to increased temperature Responses to inputs of allochthonous organic matter Objectives 14 Material and Methods 14 Paper I Paper II and III Paper IV Results and Discussion 18 Effect of temperature and nutrient availability on heterotrophic bacteria Influence of food web length and labile DOC on pelagic productivity and FWE Consequences of changes in inputs of ADOM and temperature for pelagic productivity and FWE Control of pelagic productivity, FWE and ecosystem trophic balance by colored DOC Conclusion and future perspectives 21 Author contributions 23 Acknowledgements 23 Thanks 24 References 25 4 Abstract Global records of temperature show a warming trend both in the atmosphere and in the oceans. Current climate change scenarios indicate that global temperature will continue to increase in the future. The effects will however be very different in different geographic regions. In northern Europe precipitation is projected to increase along with temperature. -

Microbial Loop' in Stratified Systems
MARINE ECOLOGY PROGRESS SERIES Vol. 59: 1-17, 1990 Published January 11 Mar. Ecol. Prog. Ser. 1 A steady-state analysis of the 'microbial loop' in stratified systems Arnold H. Taylor, Ian Joint Plymouth Marine Laboratory, Prospect Place, West Hoe, Plymouth PLl 3DH, United Kingdom ABSTRACT. Steady state solutions are presented for a simple model of the surface mixed layer, which contains the components of the 'microbial loop', namely phytoplankton, picophytoplankton, bacterio- plankton, microzooplankton, dissolved organic carbon, detritus, nitrate and ammonia. This system is assumed to be in equilibrium with the larger grazers present at any time, which are represented as an external mortality function. The model also allows for dissolved organic nitrogen consumption by bacteria, and self-grazing and mixotrophy of the microzooplankton. The model steady states are always stable. The solution shows a number of general properties; for example, biomass of each individual component depends only on total nitrogen concentration below the mixed layer, not whether the nitrogen is in the form of nitrate or ammonia. Standing stocks and production rates from the model are compared with summer observations from the Celtic Sea and Porcupine Sea Bight. The agreement is good and suggests that the system is often not far from equilibrium. A sensitivity analysis of the model is included. The effect of varying the mixing across the pycnocline is investigated; more intense mixing results in the large phytoplankton population increasing at the expense of picophytoplankton, micro- zooplankton and DOC. The change from phytoplankton to picophytoplankton dominance at low mixing occurs even though the same physiological parameters are used for both size fractions. -

Aerobic Respiration
Life is based on redox • All energy generation in biological systems is due to redox (reduction-oxidation) reactions Aerobic Respiration: + - C6H12O6 + 6 H2O ==> 6 CO2 + 24 H +24 e oxidation electron donor (aka energy source) + - (O2+ 4H + 4e ==> 2H2O) x6 reduction electron acceptor --------------------------------------- C6H12O6 + 6 O2 ==> 6 CO2 + 6 H2O overall reaction (24 electrons) Types of bacterial metabolisms • While eukaryotes only reduce O2 and oxidize organic compounds, prokaryotes can use a variety of electron donors and acceptors, organic and inorganic. - • Aerobic respiration: e acceptor is O2 - • Anaerobic respiration: e acceptor is not O2 • Fermentation: e- donor and acceptor are organic molecules • Chemolithotrophy: e- donor and acceptor are inorganic molecules • Phototrophy: e- donor is light and e- acceptor is either organic or inorganic all microorganisms energy source? chemical light chemotroph phototroph carbon source? carbon source? organic organic CO CO compound 2 compound 2 chemoheterotroph chemoautotroph photoheterotroph photoautotroph e- acceptor? Nitrifying and sulfur- use H O to reduce CO ? oxidizing bacteria 2 2 green non-sulfur and O Other than O 2 2 purple non-sulfur bacteria anoxygenic oxygenic photosynthesis: photosynthesis: green sulfur and most bacteria Organic Inorganic cyanobacteria compound compound purple sulfur bacteria fermentative organism anaerobic respiration: nitrate, sulfate, Fe(III) Aerobic or anaerobic respiration Chemolithotrophy Important molecules Redox Electron Carrier: for example the -

Modeling Biogeochemical Cycles
4 Modeling Biogeochemical Cycles Henning Rodhe 4.1 Introductory Remarks spatial average (i.e., the physical size of the reservoir itself) often has a horizontal size To formulate a model is to put together pieces of approaching that of a continent, or larger, i.e., knowledge about a particular system into a > 1000 km. The time scales corresponding to this consistent pattern that can form the basis for (1) spatial average are months, or longer. This interpretation of the past history of the system means that day-to-day variations in weather and (2) prediction of the future of the system. To and ocean currents are not generally considered be credible and useful, any model of a physical, explicitly when modeling biogeochemical cycles. chemical or biological system must rely on both The advent of fast computers and the avail- scientific fundamentals and observations of the ability of detailed data on the occurrence of world around us. High-quality observational certain chemical species have made it possible data are the basis upon which our understand- to construct meaningful cycle models with a ing of the environment rests. However, observa- much smaller and faster spatial and temporal tions themselves are not very useful unless the resolution. These spatial and time scales corre- results can be interpreted in some kind of model. spond to those in weather forecast models, i.e. Thus observations and modeling go hand in down to 100 km and 1 h. Transport processes hand. (e.g., for CO2 and sulfur compounds) in the This chapter focuses on types of models used oceans and atmosphere can be explicitly to describe the functioning of biogeochemical described in such models. -

Nitrogen and Phosphorus Limitation of Oceanic Microbial Growth During Spring in the Gulf of Aqaba
Vol. 56: 227–239, 2009 AQUATIC MICROBIAL ECOLOGY Printed September 2009 doi: 10.3354/ame01357 Aquat Microb Ecol Published online August 27, 2009 Contribution to AME Special 2 ‘Progress and perspectives in aquatic primary productivity’ OPEN ACCESS Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba David J. Suggett1,*, Noga Stambler2, Ondrej Prá$il3, Zbigniew Kolber4, Antonietta Quigg5, Evaristo Vázquez-Domínguez6, Tamar Zohary7, Tom Berman7, David Iluz8, Orly Levitan2, Tracy Lawson1, Efrat Meeder9,10, Boaz Lazar9,10, Edo Bar-Zeev2, Hana Medova3, Ilana Berman-Frank8 1Department of Biological Sciences, University of Essex, Colchester CO4 3SQ, UK 2Department of Geography and Environment, Bar-Ilan University, Ramat-Gan 52900, Israel 3Photosynthesis Laboratory, Institute of Microbiology ASCR, Opatovicky mlyn, 379 81 Trˇ ebonˇ, Czech Republic 4Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, California 95039, USA 5Department of Marine Biology and Oceanography, Texas A&M University, Galveston, Texas 77551, USA 6CSIC, Institut de Ciències del Mar, Passeig Marítim de la Barceloneta 39-43, 08003 Barcelona, Spain 7Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research, PO Box 447, Migdal 14950, Israel 8The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel 9The Interuniversity Institute for Marine Sciences Coral Beach, PO Box 469, 88103 Eilat, Israel 10Institute of Earth Sciences, The Hebrew University Edmond J. Safra Campus, Jerusalem 91904, Israel ABSTRACT: Bioassay experiments were performed to identify how growth of key groups within the mi- crobial community was simultaneously limited by nutrient (nitrogen and phosphorus) availability during spring in the Gulf of Aqaba’s oceanic waters. -

Developments in Aquatic Microbiology
INTERNATL MICROBIOL (2000) 3: 203–211 203 © Springer-Verlag Ibérica 2000 REVIEW ARTICLE Samuel P. Meyers Developments in aquatic Department of Oceanography and Coastal Sciences, Louisiana State University, microbiology Baton Rouge, LA, USA Received 30 August 2000 Accepted 29 September 2000 Summary Major discoveries in marine microbiology over the past 4-5 decades have resulted in the recognition of bacteria as a major biomass component of marine food webs. Such discoveries include chemosynthetic activities in deep-ocean ecosystems, survival processes in oligotrophic waters, and the role of microorganisms in food webs coupled with symbiotic relationships and energy flow. Many discoveries can be attributed to innovative methodologies, including radioisotopes, immunofluores- cent-epifluorescent analysis, and flow cytometry. The latter has shown the key role of marine viruses in marine system energetics. Studies of the components of the “microbial loop” have shown the significance of various phagotrophic processes involved in grazing by microinvertebrates. Microbial activities and dissolved organic carbon are closely coupled with the dynamics of fluctuating water masses. New biotechnological approaches and the use of molecular biology techniques still provide new and relevant information on the role of microorganisms in oceanic and estuarine environments. International interdisciplinary studies have explored ecological aspects of marine microorganisms and their significance in biocomplexity. Studies on the Correspondence to: origins of both life and ecosystems now focus on microbiological processes in the Louisiana State University Station. marine environment. This paper describes earlier and recent discoveries in marine Post Office Box 19090-A. Baton Rouge, LA 70893. USA (aquatic) microbiology and the trends for future work, emphasizing improvements Tel.: +1-225-3885180 in methodology as major catalysts for the progress of this broadly-based field. -

Diversity and Evolution of Bacterial Bioluminescence Genes in the Global Ocean Thomas Vannier, Pascal Hingamp, Floriane Turrel, Lisa Tanet, Magali Lescot, Y
Diversity and evolution of bacterial bioluminescence genes in the global ocean Thomas Vannier, Pascal Hingamp, Floriane Turrel, Lisa Tanet, Magali Lescot, Y. Timsit To cite this version: Thomas Vannier, Pascal Hingamp, Floriane Turrel, Lisa Tanet, Magali Lescot, et al.. Diversity and evolution of bacterial bioluminescence genes in the global ocean. NAR Genomics and Bioinformatics, Oxford University Press, 2020, 2 (2), 10.1093/nargab/lqaa018. hal-02514159 HAL Id: hal-02514159 https://hal.archives-ouvertes.fr/hal-02514159 Submitted on 21 Mar 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published online 14 March 2020 NAR Genomics and Bioinformatics, 2020, Vol. 2, No. 2 1 doi: 10.1093/nargab/lqaa018 Diversity and evolution of bacterial bioluminescence genes in the global ocean Thomas Vannier 1,2,*, Pascal Hingamp1,2, Floriane Turrel1, Lisa Tanet1, Magali Lescot 1,2,* and Youri Timsit 1,2,* 1Aix Marseille Univ, Universite´ de Toulon, CNRS, IRD, MIO UM110, 13288 Marseille, France and 2Research / Federation for the study of Global Ocean Systems Ecology and Evolution, FR2022 Tara GOSEE, 3 rue Michel-Ange, Downloaded from https://academic.oup.com/nargab/article-abstract/2/2/lqaa018/5805306 by guest on 21 March 2020 75016 Paris, France Received October 21, 2019; Revised February 14, 2020; Editorial Decision March 02, 2020; Accepted March 06, 2020 ABSTRACT ganisms and is particularly widespread in marine species (7–9). -

The Effects of Eutrophication on the Structure and Function of Microbial Biofilms
The Effects of Eutrophication on the Structure and Function of Microbial Biofilms David J. Van Horn1*, Cliff N. Dahm1 1UNM Department of Biology, 167 Castetter Hall MSC03 2020, 1 University of New Mexico, Albuquerque, NM 87131-0001 Abstract: Biofilms are the dominate form of microbial life in aquatic ecosystems and are responsible for performing a wide variety of ecosystem services including nutrient and organic matter processing and retention. Understanding how eutrophication impacts these communities is essential for ecosystem managers as many aquatic ecosystems are being enriched by anthropogenic activities. This study investigated the effects of eutrophication on biofilm productivity, community structure and diversity, and function. Increasing background dissolved organic carbon and nutrient concentrations by a factor of twelve increased biofilm ash free dry mass and the abundance of live cells ~ 22 and 200 fold respectively. Extracellular enzyme activities for five enzymes from the same samples showed a range of increase from ~ 300 times for phosphatase to 8000 times for N-acetylglucosaminidase. Enrichment decreased the bacterial diversity of these biofilms and resulted in a gradual shift in community structure that intensified from low to high enrichment. These data indicate that the productivity, community structure, and function of stream biofilm communities are highly responsive to eutrophication inputs. 1 Introduction: Microbes are the most abundant organisms on Earth and play a central role in all global processes. While often overlooked due to their inconspicuous nature, microbes contain as much carbon and ten times more nitrogen and phosphorus than plants (Whitman et al. 1998), possess unique metabolic pathways essential to biogeochemical cycles (Schlesinger 1997), and represent a potentially massive pool of genetic diversity that is only now being explored (Curtis et al. -

Controls and Structure of the Microbial Loop
Controls and Structure of the Microbial Loop A symposium organized by the Microbial Oceanography summer course sponsored by the Agouron Foundation Saturday, July 1, 2006 Asia Room, East-West Center, University of Hawaii Symposium Speakers: Peter J. leB Williams (University of Bangor, Wales) David L. Kirchman (University of Delaware) Daniel J. Repeta (Woods Hole Oceanographic Institute) Grieg Steward (University of Hawaii) The oceans constitute the largest ecosystems on the planet, comprising more than 70% of the surface area and nearly 99% of the livable space on Earth. Life in the oceans is dominated by microbes; these small, singled-celled organisms constitute the base of the marine food web and catalyze the transformation of energy and matter in the sea. The microbial loop describes the dynamics of microbial food webs, with bacteria consuming non-living organic matter and converting this energy and matter into living biomass. Consumption of bacteria by predation recycles organic matter back into the marine food web. The speakers of this symposium will explore the processes that control the structure and functioning of microbial food webs and address some of these fundamental questions: What aspects of microbial activity do we need to measure to constrain energy and material flow into and out of the microbial loop? Are we able to measure bacterioplankton dynamics (biomass, growth, production, respiration) well enough to edu/agouroninstitutecourse understand the contribution of the microbial loop to marine systems? What factors control the flow of material and energy into and out of the microbial loop? At what scales (space and time) do we need to measure processes controlling the growth and metabolism of microorganisms? How does our knowledge of microbial community structure and diversity influence our understanding of the function of the microbial loop? Program: 9:00 am Welcome and Introductory Remarks followed by: Peter J.