Sasakia Charonda (Apaturinae: Nymphalidae) and Its Three Related Butterfy Larvae Use Halitosis to Repel Predators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regeneration Traits of Celtis Sinensis Pers. and Aphananthe Aspera (Thunb.) Planch
Regeneration Traits of Celtis sinensis Pers. and Aphananthe aspera (Thunb.) Planch. in a Created Urban Tree Plantation approximately 20 years after construction Keizo TABATA* and Yukihiro MORIMOTO** Abstract: The groundbreaking example of creation of a tree plantation in an urban area is the “Inochi-No-Mori” project. The main goal of vegetation creation in Inochi-No-Mori is a deciduous broad-leaved forest dominated by Celtis sinensis and Aphananthe aspera. C. sinensis and A. aspera forests are thought to be the native vegetation of the Kyoto basin. For creation of C. sinensis and A. aspera plantations in urban areas, the promotion of natural regeneration of these two woody species is a necessity. To achieve this goal, an understanding of the growth characteristics of seedlings of these two species is required. To clarify the growth characteristics, recruitment, mortality and relative height growth rates of seedlings of C. sinensis and A. aspera in Inochi- No-Mori, we set up 163 quadrats (163 m2) on the forest floor of this tree plantation. As a result, there were no significance differences in mortality rates between two species. Recruitment rates of A. aspera seedlings, were relatively low. But low mortality and high growth rates were observed in this species. Although there were high recruitment rates in the C. sinensis seedlings, mortality rates were high and growth rates were relatively low. In a created urban tree plantation, the regeneration processes of the closely related species C. sinensis and A. aspera differed. Key Words: created urban tree plantation, growth characteristic, woody seedling succession. The main goal of vegetation creation in INTRODUCTION Inochi-No-Mori is a deciduous broad-leaved forest dominated by Celtis sinensis Pers. -

Amendment to the List of Regulated Living Organisms Under the Invasive Alien Species Act
Amendment to the List of Regulated Living Organisms under the Invasive Alien Species Act Before Animal Kingdom Living Organisms Required to have a Invasive Alien Species Uncategorized Alien Species Certificate Attached during their importation in Class Order Family Genus (IAS) (UAS) order to verify their types (LORCA) Any species of the genus Branta excluding Canada goose Canada goose (B. canadensis ) Aleutian Canada Goose (B. Anseriformes Anatidae Branta Any species of the genus Branta ( B. canadensis ) huhutchinsii. leucopareia ), Cackling Canada Goose (Branta h. minima ), Brent goose (Branta h. bernicla ) Laughing thrushes (G. canorus ) Aves Garrulax White-browed laughingthrush (G. sannio ) Any species of the family Timaliidae Masked laughingthrush excluding laughing thrushes (G. canorus ), Passeriformes Timaliidae (G. perspicillatus ) whitebrowed laughingthrush (G. sannio ), Any species of the family Timaliidae masked laughingthrush (G. perspicillatus ), Red-billed mesia Leiothrix and redbilled mesia (L. lutea ) (L. lutea ) All other genera of None Timaliidae Any species of the families Any species of the genus Cheirotonus Bolboceratidae Cheirotonus excluding Yanbaru Long-armed scarab None Ceratocanthidae (C. jambar ) Diphyllostomatidae Geotrupidae Glaphyridae Glaresidae Coleoptera Scarabaeidae Euchirus Any species of the genus Euchirus None Hybosoridae Lucanidae Ochodaeidae Passalidae Plecomidae Propomacrus Any species of the genus Propomacrus None Scarabaeidae Trogidae Any species of the genus Bombus excluding B. ardens ardens B. ardens sakagamii B. ardens tsushimanus B. beaticola beaticola B. beaticola moshkarareppus B. beaticola shikotanensis B. consobrinus wittenburgi B. deuteronymus deuteronymus B. deuteronymus maruhanabachi B. diversus diversus Insecta B. diversus tersatus B. florilegus Large earth bumblebee Apidae Bombus B. honshuensis honshuensis Any species of the genus Bombus (B. terrestris ) B. honshuensis tkalcui B. -

Evolution of Insect Color Vision: from Spectral Sensitivity to Visual Ecology
EN66CH23_vanderKooi ARjats.cls September 16, 2020 15:11 Annual Review of Entomology Evolution of Insect Color Vision: From Spectral Sensitivity to Visual Ecology Casper J. van der Kooi,1 Doekele G. Stavenga,1 Kentaro Arikawa,2 Gregor Belušic,ˇ 3 and Almut Kelber4 1Faculty of Science and Engineering, University of Groningen, 9700 Groningen, The Netherlands; email: [email protected] 2Department of Evolutionary Studies of Biosystems, SOKENDAI Graduate University for Advanced Studies, Kanagawa 240-0193, Japan 3Department of Biology, Biotechnical Faculty, University of Ljubljana, 1000 Ljubljana, Slovenia; email: [email protected] 4Lund Vision Group, Department of Biology, University of Lund, 22362 Lund, Sweden; email: [email protected] Annu. Rev. Entomol. 2021. 66:23.1–23.28 Keywords The Annual Review of Entomology is online at photoreceptor, compound eye, pigment, visual pigment, behavior, opsin, ento.annualreviews.org anatomy https://doi.org/10.1146/annurev-ento-061720- 071644 Abstract Annu. Rev. Entomol. 2021.66. Downloaded from www.annualreviews.org Copyright © 2021 by Annual Reviews. Color vision is widespread among insects but varies among species, depend- All rights reserved ing on the spectral sensitivities and interplay of the participating photore- Access provided by University of New South Wales on 09/26/20. For personal use only. ceptors. The spectral sensitivity of a photoreceptor is principally determined by the absorption spectrum of the expressed visual pigment, but it can be modified by various optical and electrophysiological factors. For example, screening and filtering pigments, rhabdom waveguide properties, retinal structure, and neural processing all influence the perceived color signal. -

Region of Nymphalidae and Libytheidae (Lepidoptera) from Japan
MORPHOLOGICAL STUDIES ON THE OCCIPITAL REGION OF NYMPHALIDAE AND LIBYTHEIDAE (LEPIDOPTERA) FROM JAPAN Takashi TSUBUKI and Nagao KOYAMA * .h2monji junior High School, 10-33, Kita6tsz{ha 1, Toshima-feu, Toleyo (170), 1opan ** Biological Laboratory, Shinshi2 Udeiversity, Cllada (386), 14pan CONTENTS I. Introduction・--・ny・・・・・・・・・・・・・・・・・-・-・・・・・・-・---・--t・-・---・ny・・・・-・-・・・-・-・・-・・・1 II. Materials and methods・-・・・・・・・・・t・・・・・・t・・・・・・・・・・・・・・`・・・・・・・・・・-・・・-・・・・・・-d・・・・・・・・・・・・2 III. General morphology of the occipital region ・・・・・・}・・・・・・・・・・・・・・・・・・・・・・・・・・・・・4 IV. Occipital structure of each species in Nymphaliclae and Libytheidae ・-ib-・・・・-・・・-・ny・・・・・--・-・・・・・・・-・・-・----・b-・・・----・-・・・-・・-・--・・-6 V. Phylogenical grouping of Nymphalidae based on the occipital structure・t・・・・・・・--・--・・-・-・・・・・・・・・・-・・・・-ny-・・・ny-・--・-----・H"""""".","".."."lg VI. Summary ・・・・-・-・-・・・・・・-・・----・・・-・・・・-・-・-・-----・・-------・-・----・23 VII. Literatures cited ・・・--・・・・・・・・・`・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・--・-・・-・・・・・・tt・・・・・・23 I. INTRODUCTION A considerable number of studies have been carried out on the occipital morphology of moths since YAGI and KoYAMA's report of 1963 (KoYAMA and MlyATA, 1969, 1970, 1975;MIYATA, 1971, 1972, 1973, 1974, 1975;MIyATA and KOYAMA, 1971, 1972, 1976). In the butterfiies, however, few papers were available concerning the occipital region, on which EHRucH (1958, a b), YAGI and KoyAMA (1963) and KoyAMA and OGAwA (1972) briefly described. Then, tried to study the occipital region of butterflies, the authors observed it preliminarily (TsuBuKI et aL 1975). The present paper deals with the occipital structure of Nymphalidae and Libytheidae from Japan with reference to its bearing on systematics. Before going further, the authors wish to express their gratitude to Prof. Dr. H. SAwADA and Prof. Dr. N. GOKAN, Tokyo University of Agriculture, for their advice and assistance. Thanks are also due to Mr. N. K6DA who gave valuable materials for this work, and to Mr. Y. YAGucm and the members of JEOL Ltd. -
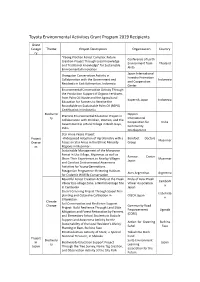
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

Issn 0972- 1800
ISSN 0972- 1800 VOLUME 22, NO. 2 QUARTERLY APRIL-JUNE, 2020 Date of Publication: 28th June, 2020 BIONOTES A Quarterly Newsletter for Research Notes and News On Any Aspect Related with Life Forms BIONOTES articles are abstracted/indexed/available in the Indian Science Abstracts, INSDOC; Zoological Record; Thomson Reuters (U.S.A); CAB International (U.K.); The Natural History Museum Library & Archives, London: Library Naturkundemuseum, Erfurt (Germany) etc. and online databases. Founder Editor Manuscripts Dr. R. K. Varshney, Aligarh, India Please E-mail to [email protected]. Board of Editors Guidelines for Authors Peter Smetacek, Bhimtal, India BIONOTES publishes short notes on any aspect of biology. Usually submissions are V.V. Ramamurthy, New Delhi, India reviewed by one or two reviewers. Jean Haxaire, Laplune, France Kindly submit a manuscript after studying the format used in this journal Vernon Antoine Brou, Jr., Abita Springs, (http://www.entosocindia.org/). Editor U.S.A. reserves the right to reject articles that do not Zdenek F. Fric, Ceske Budejovice, Czech adhere to our format. Please provide a contact Republic telephone number. Authors will be provided Stefan Naumann, Berlin, Germany with a pdf file of their publication. R.C. Kendrick, Hong Kong SAR Address for Correspondence Publication Policy Butterfly Research Centre, Bhimtal, Information, statements or findings Uttarakhand 263 136, India. Phone: +91 published are the views of its author/ source 8938896403. only. Email: [email protected] From Volume 21 Published by the Entomological Society of India (ESI), New Delhi (Nodal Officer: V.V. Ramamurthy, ESI, New Delhi) And Butterfly Research Centre, Bhimtal Executive Editor: Peter Smetacek Assistant Editor: Shristee Panthee Butterfly Research Trust, Bhimtal Published by Dr. -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

Lepidoptera: Nymphalidae
The LepidopterologicalSocietyLepidopterological Society of Japan eeLwt 7)izns. gopid. Soc. ]mpan 47 (4): Z78-284, December 1996 Ice-inoculation avoidance in hibernating larya of Apatura metis (Lepidoptera : Nymphalidae) Kazuo HosHIKAWA Faculty of Life and Environmental Science, Shimane University, Nishikawatsu, Matsue City, 690 Japan m320C Abstract Larvae of APatuva metis hibernating in Hokkaido froze at spontaneously, -70C while they were frozen at when ice-inocurated. Although the frozen larvae toleratecl -19'C down to for 24 hrs, yet they avoided inoculative freezing by two ways: 1) to prevent water invasion onto ventraHntegument, through which ice-inoculation occurred, by lateral hairs and silken mat, 2) to choose hibernating site on east-northern surface of host tree trunk, where inoculation should hardly occur. The larvae accumulated glycerol in haemolymph to the amount of 6.5% of fresh body weight, together with O.5% trehalose. For comparison, cold resistance in larvae of Sasakia charondu hibernating in Iitter layer was also studied. They showed no inoculation avoidance de$pite re]atively weak ability of freezing resistance, and cuntained trehalose (1.4%) but no glycerol. Key words Cold resistance, ice-inoculation avoidanee, hibernation, APatum metis, SUsakia charondke. Introduction Most insects hibernating above the ground in temperate or cold regions are exposed to coldness, more or Iess, hence they have developed abirities for cold resistance. Apart from co]d avoidance by migration or microhabitat selection (Hoshikawa et al., 1988), cold resistance has been studied along two lines : supercooling and freezing resistance (Asahina, 1969; Tsumuki, 1988; Leather et al,, 1993). And once acquired enough cold resistance, hibernating period is regarded as relatively safe and stable season in terms of population dynamics (Sakagami et al., 1984). -

CBD First National Report
FIRST NATIONAL REPORT OF THE REPUBLIC OF SERBIA TO THE UNITED NATIONS CONVENTION ON BIOLOGICAL DIVERSITY July 2010 ACRONYMS AND ABBREVIATIONS .................................................................................... 3 1. EXECUTIVE SUMMARY ........................................................................................... 4 2. INTRODUCTION ....................................................................................................... 5 2.1 Geographic Profile .......................................................................................... 5 2.2 Climate Profile ...................................................................................................... 5 2.3 Population Profile ................................................................................................. 7 2.4 Economic Profile .................................................................................................. 7 3 THE BIODIVERSITY OF SERBIA .............................................................................. 8 3.1 Overview......................................................................................................... 8 3.2 Ecosystem and Habitat Diversity .................................................................... 8 3.3 Species Diversity ............................................................................................ 9 3.4 Genetic Diversity ............................................................................................. 9 3.5 Protected Areas .............................................................................................10 -

Photonic Crystal Structure of Wing Scales in Sasakia Charonda Butterflies
Materials Transactions, Vol. 51, No. 2 (2010) pp. 202 to 208 Special Issue on Development and Fabrication of Advanced Materials Assisted by Nanotechnology and Microanalysis #2010 The Japan Institute of Metals Photonic Crystal Structure of Wing Scales in Sasakia Charonda Butterflies Jirˇina Mateˇjkova´-Plsˇkova´1, Dalibor Jancˇik1, Miroslav Masˇla´nˇ1, Satoshi Shiojiri2 and Makoto Shiojiri3;* 1Centre for Nanomaterial Research, Faculty of Science, Palacky University in Olomouc, Slechtitelu 11, 783 71 Olomouc, Czech Republic 2Matsubara Junior High-school, Kyoto 604-8812, Japan 3Professor Emeritus of Kyoto Institute of Technology, 1-297 Wakiyama, Kyoto 618-0091, Japan The hindwings of the male Sasakia charonda charonda butterflies comprise iridescent purple-blue areas, iridescent white-pearl areas, yellow spots and red spots as well as brown background. We have examined the microstructure of their scales by scanning electron microscopy, for applying their photonic crystal structures to fine light manipulators such as reflection elements in laser diodes. The scales in the yellow spots, red spots and brown background have almost the same structure, which is an optical diffraction grating made of ridges with two cuticle layers. Their difference comes from the contained pigments. The scales in the iridescent purple-blue and white-pearl are also the same in structure. They have seven tilted cuticle layers lapped on the ridges, which also constitute a grating. The widths of the ridge and groove in the grating are different between scales of the two kinds. It is shown that the vivid iridescence is mainly attributed to multiple interferences caused between rays reflected from the seven cuticle layers with air gaps. -
Interspecific Variation in Competitor Avoidance and Foraging Success in Sap-Attracted Insects
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/270496969 Interspecific variation in competitor avoidance and foraging success in sap- attracted insects Article in European Journal of Entomology · November 2009 DOI: 10.14411/eje.2009.066 CITATIONS READS 0 10 1 author: Jiichiro Yoshimoto University of the Valley of Guatemala 12 PUBLICATIONS 58 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Climate change effects on the biodiversity of the seasonally dry tropical forests of Motagua Valley in Guatemala View project All content following this page was uploaded by Jiichiro Yoshimoto on 28 January 2019. The user has requested enhancement of the downloaded file. Eur. J. Entomol. 106: 529–533, 2009 http://www.eje.cz/scripts/viewabstract.php?abstract=1484 ISSN 1210-5759 (print), 1802-8829 (online) Interspecific variation in competitor avoidance and foraging success in sap-attracted insects JIICHIRO YOSHIMOTO* Laboratory of Insect Ecology, Graduate School of Agriculture, Kyoto University, Kitashirakawa Oiwake-cho, Sakyo-ku, Kyoto 606-8502, Japan Key words. Aggressive interactions, community, foraging strategy, interference competition, resources, tree sap Abstract. Many insect species attracted to fermenting sap often fight for access to this resource, which results in the establishment of interspecific dominance hierarchies. In one such system, the hornet Vespa mandarinia (Hymenoptera: Vespidae) behaviourally dominates during the daytime and several subordinate species avoid aggressive interactions in various ways. In order to elucidate the interspecific variation in competitor-avoidance behaviour and its subsequent effect on foraging success, the behaviour of species of hornets, beetles and butterflies at patches (exudation spots) in Japan was recorded. -

Lepidoptera: Noctuoidea: Erebidae) and Its Phylogenetic Implications
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 113: 558–570, 2016 http://www.eje.cz doi: 10.14411/eje.2016.076 ORIGINAL ARTICLE Characterization of the complete mitochondrial genome of Spilarctia robusta (Lepidoptera: Noctuoidea: Erebidae) and its phylogenetic implications YU SUN, SEN TIAN, CEN QIAN, YU-XUAN SUN, MUHAMMAD N. ABBAS, SAIMA KAUSAR, LEI WANG, GUOQING WEI, BAO-JIAN ZHU * and CHAO-LIANG LIU * College of Life Sciences, Anhui Agricultural University, 130 Changjiang West Road, Hefei, 230036, China; e-mails: [email protected] (Y. Sun), [email protected] (S. Tian), [email protected] (C. Qian), [email protected] (Y.-X. Sun), [email protected] (M.-N. Abbas), [email protected] (S. Kausar), [email protected] (L. Wang), [email protected] (G.-Q. Wei), [email protected] (B.-J. Zhu), [email protected] (C.-L. Liu) Key words. Lepidoptera, Noctuoidea, Erebidae, Spilarctia robusta, phylogenetic analyses, mitogenome, evolution, gene rearrangement Abstract. The complete mitochondrial genome (mitogenome) of Spilarctia robusta (Lepidoptera: Noctuoidea: Erebidae) was se- quenced and analyzed. The circular mitogenome is made up of 15,447 base pairs (bp). It contains a set of 37 genes, with the gene complement and order similar to that of other lepidopterans. The 12 protein coding genes (PCGs) have a typical mitochondrial start codon (ATN codons), whereas cytochrome c oxidase subunit 1 (cox1) gene utilizes unusually the CAG codon as documented for other lepidopteran mitogenomes. Four of the 13 PCGs have incomplete termination codons, the cox1, nad4 and nad6 with a single T, but cox2 has TA. It comprises six major intergenic spacers, with the exception of the A+T-rich region, spanning at least 10 bp in the mitogenome.