Rapid Detection of Six Glucocorticoids Added Illegally to Dietary Supplements by Combining TLC with Spot-Concentrated Raman Scattering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -
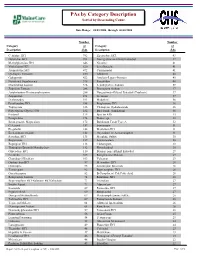
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

OHIO STATE BOARD of PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider
OHIO STATE BOARD OF PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider- TEL: 614/466-4143 E-MAIL: [email protected] FAX: 614/752-4836 TTY/TDD: Use the Ohio Relay Service: 1-800/750-0750 www.pharmacy.ohio.gov ORDER OF THE STATE BOARD OF PHARMACY (Docket No. D-031110-034) In The Matter Of: CHRISTOPHER KIEL, R.Ph. 8260 Cyrus Lane Sagamore Hills, Ohio 44067 (R.Ph. No. 03-1-12367) INTRODUCTION THE MATTER OF CHRISTOPHER KIEL CAME FOR HEARING ON MAY 4, 2004, BEFORE THE FOLLOWING MEMBERS OF THE BOARD: ROBERT P. GIACALONE, R.Ph. (presiding); DIANE C. ADELMAN, R.Ph.; GREGORY BRAYLOCK, R.Ph.; SUZANNE R. EASTMAN, R.Ph.; ELIZABETH I. GREGG, R.Ph.; LAWRENCE J. KOST, R.Ph.; NATHAN S. LIPSYC, R.Ph.; DOROTHY S. TEATER, PUBLIC MEMBER; AND JAMES E. TURNER, R.Ph. CHRISTOPHER KIEL WAS REPRESENTED BY JOSEPH W. DIEMERT, JR. AND DIANE A. CALTA AND THE STATE OF OHIO WAS REPRESENTED BY SALLY ANN STEUK, ASSISTANT ATTORNEY GENERAL. SUMMARY OF EVIDENCE State’s Witnesses 1. Joann Predina, R.Ph., Ohio State Board of Pharmacy 2. Frederick Lochner, U.S. Food and Drug Administration (FDA) Respondent's Witnesses 1. Christopher Kiel, R.Ph., Respondent State's Exhibits 1K. Copy of Notice of Opportunity For Hearing letter [11-10-03] 1A-1K-E. Procedurals 1K-F. Copy of Amendment Notice [03-03-04] 1G-1K-K. Procedurals 2. Copy of Ohio State Board of Pharmacy Compliance Bulletin 93-002 [09-15-93] 2A. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0281830 A1 Sulur Et Al
US 2011 (0281830A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0281830 A1 Sulur et al. (43) Pub. Date: Nov. 17, 2011 (54) NOVEL DERMACEUTICAL CREAM MADE (30) Foreign Application Priority Data USING SODIUM FUSDATE AND STEROIDS Jan. 21, 2009 (IN) ........................... 134/MUMA2009 (75) Inventors: Vanangamudi Subramaniam Publication Classification Sulur,r Chennai (IN);: Madhavan (51) Int. Cl. Srinivasan, Chennai (IN); A 6LX 3/575 (2006.01) Neelakandan Narayanan Chulliel, A6IP3L/00 (2006.01) Chennai (IN); Haridas Sankar, A6IP 29/00 (2006.01) Mumbai (IN). Kausik Ghosh, A6IP 700 (2006.01) Chennai (IN) (52) U.S. Cl. ........................................................ S14/17O (73) Assignee: APEX LABORATORIES (57) ABSTRACT PRIVATE LIMITED, CHENNAI, The invention discloses a dermaceutical cream containing TN (IN) steroids and an antibacterial agent in the form of Fusidic acid, which Fusidic acid is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is con (21) Appl. No.: 13/144.932 verted into Fusidic acid under oxygen-free environment. The cream of the present invention has greater shelf-life stability (22) PCT Filed: Jan. 20, 2010 and the finer particle size of the API than the conventional creams containing Fusidic acid. The cream of the present (86). PCT No.: PCT/B2O1O/OSO242 invention contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, and steroids in a cream S371 (c)(1), base comprising an acid, a co-solvent, an emulsifier and a (2), (4) Date: Jul.18, 2011 waxy material along with water, preferably purified water. US 2011/028 1830 A1 Nov. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

AMRI India Pvt
WE’VE GOT API DEVELOPMENT AND MANUFACTURING DOWN TO AN EXACT SCIENCE API Commercial Product Catalogue PRODUCT CATALOGUE API Commercial US EU Japan US EU Japan API Name Site CEP India China API Name Site CEP India China DMF DMF DMF DMF DMF DMF Rozzano Quinto de Adenosine Betaine Citrate Anhydrous Bon Encontre, France A Stampi, Italy • Betametasone-17,21- Alcaftadine Valladolid, Spain Valladolid, Spain • Dipropionate Sterile • Alclometasone-17,21- Valladolid, Spain Betamethasone Acetate Valladolid, Spain Dipropionate • • • Altrenogest Valladolid, Spain • • Betamethasone Base Valladolid, Spain Aminobisamide HCl Rensselaer, US Betamethasone Benzoate Valladolid, Spain Amphetamine Aspartate Betamethasone Valerate Rensselaer, US Valladolid, Spain Monohydrate Milled • Acetate Betamethasone-17,21- Amphetamine Sulfate Rensselaer, US Valladolid, Spain • Dipropionate • • • • Rozzano Quinto de Argatroban Betamethasone-17-Valerate Valladolid, Spain Stampi, Italy • • • • Betamethasone-21- Atenolol Aurangabad, India Valladolid, Spain • • • Phosphate Disodium Salt • • Rozzano Quinto de Bromfenac Monosodium Atracurium Besylate Lodi, Italy Stampi, Italy • Salt Sesquihydrate • • Rozzano Quinto de Atropine Sulfate Grafton, US Bromocriptine Mesylate • Stampi, Italy • • • Rozzano Quinto de Azelastine HCl Budesonide Valladolid, Spain Stampi, Italy • • • • • Rozzano Valleambrosia, Aztreonam (not sterile) Budesonide Sterile Valladolid, Spain Italy • • • • B Bamifylline HCl Bon Encontre, France • C Capecitabine Lodi, Italy • • Beclomethasone-17,21- Valladolid, Spain -

AHFS Therapeutic
AHFS Therapeutic AHFS Therapeutic Class Description HICL HICL Description Program Edit Criteria 1 Edit Criteria 2 Edit Criteria 3 Edit Criteria 4 Edit Criteria 5 Class 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 0.667000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 0.750000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 1.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 2.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 2.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 3.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 3.750000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 6.000000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE MAX DAILY UNITS : 7.500000 240408 CARDIOTONIC AGENTS 000004 DIGOXIN PACE Medical Exception Required 282032 RESPIRATORY AND CNS STIMULANTS 000007 CAFFEINE/DEXTROSE PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000008 CAFFEINE/MULTIVITAMIN PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000009 CAFFEINE/ETHYL ALCOHOL PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000010 CAFFEINE/SODIUM BENZOATE PACE Cannnot co‐administer inhaled Insulin and Asthma/COPD/Smoking Cessation agents 282032 RESPIRATORY AND CNS STIMULANTS 000011 CAFFEINE CITRATE -

EUROPEAN PHARMACOPOEIA 10.0 Index 1. General Notices
EUROPEAN PHARMACOPOEIA 10.0 Index 1. General notices......................................................................... 3 2.2.66. Detection and measurement of radioactivity........... 119 2.1. Apparatus ............................................................................. 15 2.2.7. Optical rotation................................................................ 26 2.1.1. Droppers ........................................................................... 15 2.2.8. Viscosity ............................................................................ 27 2.1.2. Comparative table of porosity of sintered-glass filters.. 15 2.2.9. Capillary viscometer method ......................................... 27 2.1.3. Ultraviolet ray lamps for analytical purposes............... 15 2.3. Identification...................................................................... 129 2.1.4. Sieves ................................................................................. 16 2.3.1. Identification reactions of ions and functional 2.1.5. Tubes for comparative tests ............................................ 17 groups ...................................................................................... 129 2.1.6. Gas detector tubes............................................................ 17 2.3.2. Identification of fatty oils by thin-layer 2.2. Physical and physico-chemical methods.......................... 21 chromatography...................................................................... 132 2.2.1. Clarity and degree of opalescence of -

Drugs and Health Products
Notice: Prescription Drug List (PDL): Fluticasone propionate http://www.hc-sc.gc.ca/dhp-mps/consultation/drug-medic/pdl_ldo_consul... Home > Drugs & Health Products > Public Involvement & Consultations > Drug Products Drugs and Health Products Notice: Prescription Drug List (PDL): Fluticasone propionate November 27, 2015 Our file number: 15-112766-443 The purpose of this Notice of Consultation is to provide an opportunity to comment on the proposal to revise the listing for Adrenocortical Hormones or their salts or derivatives on the Human Prescription Drug List (PDL) to permit the non-prescription use of Fluticasone propionate for the conditions listed below. Only the human part of the PDL is proposed to be revised. The proposed qualifier for the listing on the Human List is: Drugs containing the following: Adrenocortical hormones or their salts or derivatives Including (but not limited to): Betamethasone valerate, betamethasone sodium, betamethasone phosphate, betamethasone dipropionate, budesonide, ciclesonide, clobetasone, cortisone, dexamethasone sodium, dexamethasone phosphate, dexamethasone acetate, difluprednate, fludrocortisone acetate, flunisolide, fluticasone propionate, fluticasone furoate, hydrocortisone acetate, hydrocortisone aceponate, hydrocortisone sodium, methylprednisolone acetate, methylprednisolone, methylprednisolone succinate, methylprednisolone sodium, mometasone furoate, prednisolone acetate, prednisolone sodium, prednisolone phosphate, prednisone, triamcinolone acetonide, triamcinolone hexacetonide Qualifier: -

Joint Injection Workshop
Joint Injection Workshop Dr Laurel Young Redcliffe and Northside Rheumatology (RNR) Joint injections • Indications • Type of steroid and why • Technique • Complications • Consent – verbal, written • Imaging? Indications for aspirating or injecting joints/soft tissues Reasons for joint aspirations Reasons for injections Exclude infection (mandatory) Diagnostic – eg. LA Therapeutic Diagnostic e.g crystals, (relieve symptoms) inflammation Joints, tendon sheaths, bursa, CTS What to inject? Type of steroid Duration in joint - solubility Corticosteroid Solubility Cost Betamethasone sodium phosphate Most soluble Dexamethasone sodium phosphate Soluble Prednisone sodium phosphate Soluble Methylprednisolone acetate Slightly soluble $4.72 (40mg/vial) Betamethasone sodium phosphate + Slightly soluble $5.34 Bethamethasone acetate (2.96/2.71mg/vial) Triamcinolone diacetate Slightly soluble Prednisolone tebutate Slightly soluble Trimancinolone acetonide Relatively insoluble $27.25 (10mg/vial) $81.39 (40mg/vial) Triamcinolone hexacetonide Relatively insoluble Hydrocortisone acetate Relatively insoluble Dexamethasone acetate Relatively insoluble Fluorinated vs non-fluorinated steroids Methylprednisolone acetate Triamcinolone acetonide Betamethasone Fluorinated steroids risk in s/c injections – fat necrosis, hypopigmentation Corticosteroid Fluorinated Betamethasone sodium phosphate Yes Betamethasone sodium Yes phosphoate/Betamethasone acetate Dexamethasone sodium phosphate Yes Triamcinolone diacetate Yes Trimancinolone acetonide Yes Triamcinolone hexacetonide -

Triamcinolone Aqueous Suspensions After Autoclaving
US 20060094700A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0094700 A1 Lyons (43) Pub. Date: May 4, 2006 (54) HEAT STERILIZATION OF A STEROID IN (22) Filed: Nov. 2, 2004 THE PRESENCE OF PHOSPHATE Publication Classi?cation (75) Inventor: Robert T. Lyons, Laguna Hills, CA (Us) (51) Int. Cl. A61K 31/5 73 (2006.01) Correspondence Address: (52) US. Cl. ............................................................ .. 514/179 ALLERGAN, INC., LEGAL DEPARTMENT 2525 DUPONT DRIVE, T2-7H (57) ABSTRACT IRVINE CA 92612-1599 US a ( ) A process for sterilizing a Water-insoluble steroid composi (73) Assigneej ALLERGAN, INC" Irvine’ C A (Us) tion comprising heat sterilizing the steroid in the presence of phosphate is disclosed herein. Compositions related thereto (21) Appl. No.: 10/980,026 are also disclosed. Effect of Additives on Particle Size Distribution of 20% Triamcinolone Aqueous Suspensions After Autoclaving 25 l I I l E -Mean =- zo- E9001 P'tile . 1:“ .2 ‘I 5 \ g 15- - a .2 n g 10- . (D 2 .2 t 5- - N n- I c I Non-Aut'oclaved Autocl I+ None Autocl'+ NaCl Autocl + NaCl + PQ Additives to Triamcinolone Acetonide Suspension Patent Application Publication May 4, 2006 US 2006/0094700 A1 Figure 1 Effect of Additives on Particle Size Distribution of 20% Triamcinolone Aqueous Suspensions After Autoclaving a: l I I l ‘v E -Mean =5 20- 90th P'tile . .5 ‘I g \ I: 15" ' H .2 D .8 10- - w 2 .2 ‘E 5- - N n- o I Non-Aut'oclaved Autocl I+ None Autocl'+ NaCl Autocl + NaCl + Pq Additives to Triamcinolone Acetonide Suspension US 2006/0094700 A1 May 4, 2006 HEAT STERILIZATION OF A STEROID IN THE presence of phosphate is disclosed herein.