Stability of Betamethasone Esters in Some Topical Dosage Forms and Its Impact on Their Biological Potential
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Betamethasone Valerate Foam: a Look at the Clinical Data
Review: Clinical Trial Outcomes Betamethasone valerate foam: a look at the clinical data Clin. Invest. (2014) 4(3), 259–267 Topical corticosteroids and especially betamethasone valerate (BMV) have Avner Shemer1, Nicole Sakka1 & been used topically to relieve many inflammatory skin conditions such as Dov Tamarkin*2 psoriasis and atopic dermatitis. The vehicle used to deliver topical drugs 1Department of Dermatology, the Chaim Sheba Medical Center, Affiliated with the can influence the performance of these topical applications. BMV has Tel-Aviv University, Sackler School of Medicine, traditionally been available in creams, ointments, lotions and sprays. In Tel Hashomer, Israel the early 2000s, a topical hydroethanolic BMV foam became commercially 2Foamix Ltd., 2 Holzman Street, Weizmann available. Subsequently, alcohol-free emulsion- and petrolatum-based Science Park, Rehovot 76704, Israel foam formulations were also developed. This manuscript reviews the *Author for correspondence: Tel.: +972 52 457 5677 properties of BMV foams and clinical studies that have been conducted Fax: +972 8 853 1102 to assess their efficacy and safety as treatments for scalp and non-scalp [email protected] psoriasis, as well as other dermatological inflammatory conditions. Keywords: betamethasone valerate • foam • psoriasis • topical corticosteroids Topical corticosteroids have been ranked in four groups consisting of seven classes ranging from ultra-high potency preparations (class 1) to low-potency prepara- tions (class 7). Betamethasone valerate (BMV) is a mid-potency corticosteroid (class 3–5, depending on the dosage form), used topically to relieve inflammatory skin conditions. It is used as a treatment for psoriasis, atopic dermatitis and other corticosteroid-responsive dermatoses. The vehicle used to deliver topical drugs can influence the performance of these drugs. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Albany-Molecular-Research-Regulatory
PRODUCT CATALOGUE API COMMERCIAL US EU Japan US EU Japan API Name Site CEP India API Name Site CEP India DMF DMF DMF DMF DMF DMF A Abiraterone Malta • Benztropine Mesylate Cedarburg • Adenosine Rozzano - Quinto de' Stampi • • * Betaine Citrate Anhydrous Bon Encontre • Betametasone-17,21- Alcaftadine Spain Spain • • Dipropionate Sterile • Alclometasone-17, 21- Spain Betamethasone Acetate Spain Dipropionate • • Altrenogest Spain • • Betamethasone Base Spain Amphetamine Aspartate Rensselaer Betamethasone Benzoate Spain * Monohydrate Milled • Betamethasone Valerate Amphetamine Sulfate Rensselaer Spain * • Acetate Betamethasone-17,21- Argatroban Rozzano - Quinto de' Stampi Spain • • Dipropionate • • • Atenolol India • • Betamethasone-17-Valerate Spain • • Betamethasone-21- Atracurium Besylate Rozzano - Quinto de' Stampi Spain • Phosphate Disodium Salt • • Bromfenac Monosodium Atropine Sulfate Cedarburg Lodi * • Salt Sesquihydrate • • Azanidazole Lodi Bromocriptine Mesylate Rozzano - Quinto de' Stampi • • • • • Azelastine HCl Rozzano - Quinto de' Stampi • • Budesonide Spain • • Aztreonam Rozzano - Valle Ambrosia • • Budesonide Sterile Spain • • B Bamifylline HCl Bon Encontre • Butorphanol Tartrate Cedarburg • Beclomethasone-17, 21- Spain Capecitabine Lodi Dipropionate • C • 2 *Please contact our Accounts Managers in case you are interested in this API. 3 PRODUCT CATALOGUE API COMMERCIAL US EU Japan US EU Japan API Name Site CEP India API Name Site CEP India DMF DMF DMF DMF DMF DMF Dexamethasone-17,21- Carbimazole Bon Encontre Spain • Dipropionate -

Role of Corticosteroids in Treatment of Vitiligo
Chapter 9 Role of Corticosteroids in Treatment of Vitiligo Nooshin Bagherani Additional information is available at the end of the chapter http://dx.doi.org/10.5772/48384 1. Introduction Vitiligo is the most frequent pigmentary disorder ( Bagherani et al., 2011; Nazer et al., 2011;Yaghoobi et al., 2011a; as cited in Wolff et al., 2007). It is an acquired, idiopathic and progressive skin disease (Bagherani et al., 2011; Shameer et al., 2005; Yaghoobi et al., 2011a), characterized by sharply demarcated depigmented lesions on any part of the body (Van Geel et al., 2004). This disease can also affect hair and mucosal areas such as mouth and genitalia (Gawkrodger et al., 2010). Vitiligo usually begins after birth (Gawkrodger et al., 2010). Regarding the studies retrieved from PubMed since 1995, it has been shown that approximately 50% of the vitiligo cases have its onset before the age of 20 years and 25% before the age of 14 years (Kakourou, 2009). The incidence rate of vitiligo is between 0.1-2% of the world population ( Bagherani et al., 20011; Yaghoobi et al., 2011a,b; as cited in Alkhateeb et al., 2003). Its incidence in those with racially pigmented skin is higher, although reliable figures are not available (Burns et al., 2004; Howitz et al., 1977) . The prevalence has been reported as high as 4% in some South Asian, Mexican and American populations ( Parsad et al., 2003; Sehgal & Srivastava, 2007; Szczurko & Boon, 2008). Both sexes are equally afflicted by vitiligo (Krϋger et al., 2011; Njoo & Westerhof, 2001; Wolf et al.,2007) . In some studies, a female preponderance has been reported ( Burns at al., 2004; Howitz et al. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -
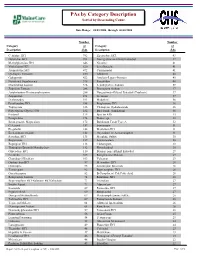
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

OHIO STATE BOARD of PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider
OHIO STATE BOARD OF PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider- TEL: 614/466-4143 E-MAIL: [email protected] FAX: 614/752-4836 TTY/TDD: Use the Ohio Relay Service: 1-800/750-0750 www.pharmacy.ohio.gov ORDER OF THE STATE BOARD OF PHARMACY (Docket No. D-031110-034) In The Matter Of: CHRISTOPHER KIEL, R.Ph. 8260 Cyrus Lane Sagamore Hills, Ohio 44067 (R.Ph. No. 03-1-12367) INTRODUCTION THE MATTER OF CHRISTOPHER KIEL CAME FOR HEARING ON MAY 4, 2004, BEFORE THE FOLLOWING MEMBERS OF THE BOARD: ROBERT P. GIACALONE, R.Ph. (presiding); DIANE C. ADELMAN, R.Ph.; GREGORY BRAYLOCK, R.Ph.; SUZANNE R. EASTMAN, R.Ph.; ELIZABETH I. GREGG, R.Ph.; LAWRENCE J. KOST, R.Ph.; NATHAN S. LIPSYC, R.Ph.; DOROTHY S. TEATER, PUBLIC MEMBER; AND JAMES E. TURNER, R.Ph. CHRISTOPHER KIEL WAS REPRESENTED BY JOSEPH W. DIEMERT, JR. AND DIANE A. CALTA AND THE STATE OF OHIO WAS REPRESENTED BY SALLY ANN STEUK, ASSISTANT ATTORNEY GENERAL. SUMMARY OF EVIDENCE State’s Witnesses 1. Joann Predina, R.Ph., Ohio State Board of Pharmacy 2. Frederick Lochner, U.S. Food and Drug Administration (FDA) Respondent's Witnesses 1. Christopher Kiel, R.Ph., Respondent State's Exhibits 1K. Copy of Notice of Opportunity For Hearing letter [11-10-03] 1A-1K-E. Procedurals 1K-F. Copy of Amendment Notice [03-03-04] 1G-1K-K. Procedurals 2. Copy of Ohio State Board of Pharmacy Compliance Bulletin 93-002 [09-15-93] 2A. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0281830 A1 Sulur Et Al
US 2011 (0281830A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0281830 A1 Sulur et al. (43) Pub. Date: Nov. 17, 2011 (54) NOVEL DERMACEUTICAL CREAM MADE (30) Foreign Application Priority Data USING SODIUM FUSDATE AND STEROIDS Jan. 21, 2009 (IN) ........................... 134/MUMA2009 (75) Inventors: Vanangamudi Subramaniam Publication Classification Sulur,r Chennai (IN);: Madhavan (51) Int. Cl. Srinivasan, Chennai (IN); A 6LX 3/575 (2006.01) Neelakandan Narayanan Chulliel, A6IP3L/00 (2006.01) Chennai (IN); Haridas Sankar, A6IP 29/00 (2006.01) Mumbai (IN). Kausik Ghosh, A6IP 700 (2006.01) Chennai (IN) (52) U.S. Cl. ........................................................ S14/17O (73) Assignee: APEX LABORATORIES (57) ABSTRACT PRIVATE LIMITED, CHENNAI, The invention discloses a dermaceutical cream containing TN (IN) steroids and an antibacterial agent in the form of Fusidic acid, which Fusidic acid is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is con (21) Appl. No.: 13/144.932 verted into Fusidic acid under oxygen-free environment. The cream of the present invention has greater shelf-life stability (22) PCT Filed: Jan. 20, 2010 and the finer particle size of the API than the conventional creams containing Fusidic acid. The cream of the present (86). PCT No.: PCT/B2O1O/OSO242 invention contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, and steroids in a cream S371 (c)(1), base comprising an acid, a co-solvent, an emulsifier and a (2), (4) Date: Jul.18, 2011 waxy material along with water, preferably purified water. US 2011/028 1830 A1 Nov.