2020 PROVIDENCE FORMULARY a Updates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

25-CRNVMS3-COPPER.Pdf
EXCERPTED FROM: Vitamin and Mineral Safety 3rd Edition (2013) Council for Responsible Nutrition (CRN) www.crnusa.org Copper Introduction Copper, like iron and some other elements, is a transition metal and performs at least some of its functions through oxidation-reduction reactions. These reactions involve the transition from Cu1+ to Cu2+. There is little or no Cu valence 0 (the metallic form) in biological systems (European Commission, Scientific Committee on Food [EC SCF] 2003). The essential role of copper was recognized after animals that were fed only a whole-milk diet developed an apparent deficiency that did not respond to iron supplementation and was then recognized as a copper deficiency (Turnlund 1999). The similarity of copper-deficiency anemia and iron-deficiency anemia helped scientists to understand copper’s important biological role as the activator of the enzyme ferroxidase I (ceruloplasmin), which is necessary for iron absorption and mobilization from storage in the liver (Linder 1996; Turnlund 1999; EC SCF 2003). Copper activates several enzymes involved in the metabolism of amino acids and their metabolites, energy, and the activated form of oxygen, superoxide. Enzyme activation by copper produces physiologically important effects on connective tissue formation, iron metabolism, central nervous system activity, melanin pigment formation, and protection against oxidative stress. There are two known inborn errors of copper metabolism. Wilson disease results when an inability to excrete copper causes the element to accumulate, and Menkes disease results when an inability to absorb copper creates a copper deficiency (Turnlund 1994). Safety Considerations Copper is relatively nontoxic in most mammals, including humans (Scheinberg and Sternlieb 1976; Linder 1996). -

DESCRIPTION Nicadan® Tablets Are a Specially Formulated Dietary
DESCRIPTION niacinamide may reduce the hepatic metabolism of primidone Nicadan® tablets are a specially formulated dietary supplement and carbamazepine. Individuals taking these medications containing natural ingredients with anti-inflammatory properties. should consult their physician. Individuals taking anti- Each pink-coated tablet is oval shaped, scored and embossed diabetes medications should have their blood glucose levels with “MM”. Nicadan® is for oral administration only. monitored. Nicadan® should be administered under the supervision of a Allergic sensitization has been reported rarely following oral licensed medical practitioner. administration of folic acid. Folic acid above 1 mg daily may obscure pernicious anemia in that hematologic remission may INGREDIENTS occur while neurological manifestations remain progressive. Each tablet of Nicadan® contains: Vitamin C (as Ascorbic Acid).................100 mg DOSAGE AND ADMINISTRATION Niacinamide (Vitamin B-3) ..................800 mg Take one tablet daily with food or as directed by a physician. Vitamin B-6 (as Pyridoxine HCI) . .10 mg Nicadan® tablets are scored, so they may be broken in half Folic Acid...............................500 mcg if required. Magnesium (as Magnesium Citrate).............5 mg HOW SUPPLIED Zinc (as Zinc Gluconate).....................20 mg Nicadan® is available in a bottle containing 60 tablets. Copper (as Copper Gluconate)..................2 mg 43538-440-60 Alpha Lipoic Acid...........................50 mg Store at 15°C to 30°C (59°F to 86°F). Keep bottle tightly Other Ingredients: Microcrystalline cellulose, Povidone, closed. Store in cool dry place. Hypromellose, Croscarmellose Sodium, Polydextrose, Talc, Magnesium Sterate Vegetable, Vegetable Stearine, Red Beet KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF Powder, Titanium Dioxide, Maltodextrin and Triglycerides. CHILDREN. -

Regulatory News
WHO Drug Information Vol. 28, No. 4, 2014 Regulatory news Ebola curative – transfusions of whole blood or blood plasma from recovered patients Update on treatments and vaccines have been scheduled to be conducted in Liberia, in line with WHO technical The Ebola crisis has prompted an guidelines (4). unprecedented cooperation between regulators In September the European Medicines to support WHO and to advise on possible Agency (EMA) established an expert pathways for the development, evaluation and group to review available information approval of medicines to fight Ebola. Progress on Ebola experimental treatments – towards provision of treatments and vaccines is excluding convalescent therapies – and summarized below. invited developers to submit their data (5). In August 2014, a WHO-convened panel Vaccines had agreed unanimously that is ethically On 29–30 September, 70 experts acceptable to use of experimental attended a WHO-convened consultation medicines and vaccines under the on Ebola vaccines. They took stock of the exceptional circumstances of the Ebola many ongoing efforts to rapidly evaluate epidemic (1). In early September, WHO the safety and efficacy of Ebola vaccines convened a consultation on potential for deployment as soon as possible to Ebola therapies and vaccines (2). The critical frontline workers and ultimately to importance of supportive care and populations at risk in mass vaccination community response was stressed in this campaigns. Two candidate vaccines have and subsequent discussions. clinical-grade vials available for safety trials. (6) Treatments In October, WHO convened industry In September, more than 200 experts leaders and key partners to discuss trials from around the world met at WHO and production of Ebola vaccine (7). -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -
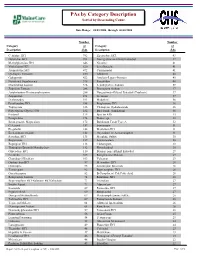
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

OHIO STATE BOARD of PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider
OHIO STATE BOARD OF PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider- TEL: 614/466-4143 E-MAIL: [email protected] FAX: 614/752-4836 TTY/TDD: Use the Ohio Relay Service: 1-800/750-0750 www.pharmacy.ohio.gov ORDER OF THE STATE BOARD OF PHARMACY (Docket No. D-031110-034) In The Matter Of: CHRISTOPHER KIEL, R.Ph. 8260 Cyrus Lane Sagamore Hills, Ohio 44067 (R.Ph. No. 03-1-12367) INTRODUCTION THE MATTER OF CHRISTOPHER KIEL CAME FOR HEARING ON MAY 4, 2004, BEFORE THE FOLLOWING MEMBERS OF THE BOARD: ROBERT P. GIACALONE, R.Ph. (presiding); DIANE C. ADELMAN, R.Ph.; GREGORY BRAYLOCK, R.Ph.; SUZANNE R. EASTMAN, R.Ph.; ELIZABETH I. GREGG, R.Ph.; LAWRENCE J. KOST, R.Ph.; NATHAN S. LIPSYC, R.Ph.; DOROTHY S. TEATER, PUBLIC MEMBER; AND JAMES E. TURNER, R.Ph. CHRISTOPHER KIEL WAS REPRESENTED BY JOSEPH W. DIEMERT, JR. AND DIANE A. CALTA AND THE STATE OF OHIO WAS REPRESENTED BY SALLY ANN STEUK, ASSISTANT ATTORNEY GENERAL. SUMMARY OF EVIDENCE State’s Witnesses 1. Joann Predina, R.Ph., Ohio State Board of Pharmacy 2. Frederick Lochner, U.S. Food and Drug Administration (FDA) Respondent's Witnesses 1. Christopher Kiel, R.Ph., Respondent State's Exhibits 1K. Copy of Notice of Opportunity For Hearing letter [11-10-03] 1A-1K-E. Procedurals 1K-F. Copy of Amendment Notice [03-03-04] 1G-1K-K. Procedurals 2. Copy of Ohio State Board of Pharmacy Compliance Bulletin 93-002 [09-15-93] 2A. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0281830 A1 Sulur Et Al
US 2011 (0281830A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0281830 A1 Sulur et al. (43) Pub. Date: Nov. 17, 2011 (54) NOVEL DERMACEUTICAL CREAM MADE (30) Foreign Application Priority Data USING SODIUM FUSDATE AND STEROIDS Jan. 21, 2009 (IN) ........................... 134/MUMA2009 (75) Inventors: Vanangamudi Subramaniam Publication Classification Sulur,r Chennai (IN);: Madhavan (51) Int. Cl. Srinivasan, Chennai (IN); A 6LX 3/575 (2006.01) Neelakandan Narayanan Chulliel, A6IP3L/00 (2006.01) Chennai (IN); Haridas Sankar, A6IP 29/00 (2006.01) Mumbai (IN). Kausik Ghosh, A6IP 700 (2006.01) Chennai (IN) (52) U.S. Cl. ........................................................ S14/17O (73) Assignee: APEX LABORATORIES (57) ABSTRACT PRIVATE LIMITED, CHENNAI, The invention discloses a dermaceutical cream containing TN (IN) steroids and an antibacterial agent in the form of Fusidic acid, which Fusidic acid is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is con (21) Appl. No.: 13/144.932 verted into Fusidic acid under oxygen-free environment. The cream of the present invention has greater shelf-life stability (22) PCT Filed: Jan. 20, 2010 and the finer particle size of the API than the conventional creams containing Fusidic acid. The cream of the present (86). PCT No.: PCT/B2O1O/OSO242 invention contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, and steroids in a cream S371 (c)(1), base comprising an acid, a co-solvent, an emulsifier and a (2), (4) Date: Jul.18, 2011 waxy material along with water, preferably purified water. US 2011/028 1830 A1 Nov. -

Management of Copper Deficiency in Cholestatic Infants / Blackmer, Bailey 2012
XXX10.1177/0884533612461531Nutrition in Clinical PracticeManagement of Copper Deficiency in Cholestatic Infants / Blackmer, Bailey 2012 Clinical Observations Nutrition in Clinical Practice Volume 28 Number 1 Management of Copper Deficiency in Cholestatic Infants: February 2013 75-86 © 2012 American Society Review of the Literature and a Case Series for Parenteral and Enteral Nutrition DOI: 10.1177/0884533612461531 ncp.sagepub.com hosted at online.sagepub.com Allison Beck Blackmer, PharmD, BCPS1; and Elizabeth Bailey, RD2 Abstract Copper is an essential trace element, playing a critical role in multiple functions in the body. Despite the necessity of adequate copper provision and data supporting the safety of copper administration during cholestasis, it remains common practice to reduce or remove copper in parenteral nutrition (PN) solutions after the development of cholestasis due to historical recommendations supporting this practice. In neonates, specifically premature infants, less is known about required copper intakes to accumulate copper stores and meet increased demands during rapid growth. Pediatric surgical patients are at high risk for hepatic injury during long-term PN provision and a balance is needed between the potential for reduced biliary excretion of copper and adequate copper intakes to prevent deficiency. Copper deficiency has been documented in several pediatric patients with cholestasis when parenteral copper was reduced or removed. Few data guide the management of copper deficiency in the pediatric population. The following case series describes our experience with successfully managing copper deficiency in 3 cholestatic infants after copper had been reduced or removed from their PN. Classic signs of copper deficiency were present, including hypocupremia, anemia, neutropenia, thrombocytopenia, and osteopenia. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

Toxicological Profile for Copper
TOXICOLOGICAL PROFILE FOR COPPER U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry September 2004 COPPER ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. COPPER iii UPDATE STATEMENT A Toxicological Profile for Copper, Draft for Public Comment was released in September 2002. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, Mailstop F-32 Atlanta, Georgia 30333 COPPER vii QUICK REFERENCE FOR HEALTH CARE PROVIDERS Toxicological Profiles are a unique compilation of toxicological information on a given hazardous substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation of available toxicologic and epidemiologic information on a substance. Health care providers treating patients potentially exposed to hazardous substances will find the following information helpful for fast answers to often-asked questions. Primary Chapters/Sections of Interest Chapter 1: Public Health Statement: The Public Health Statement can be a useful tool for educating patients about possible exposure to a hazardous substance. It explains a substance’s relevant toxicologic properties in a nontechnical, question-and-answer format, and it includes a review of the general health effects observed following exposure. Chapter 2: Relevance to Public Health: The Relevance to Public Health Section evaluates, interprets, and assesses the significance of toxicity data to human health.