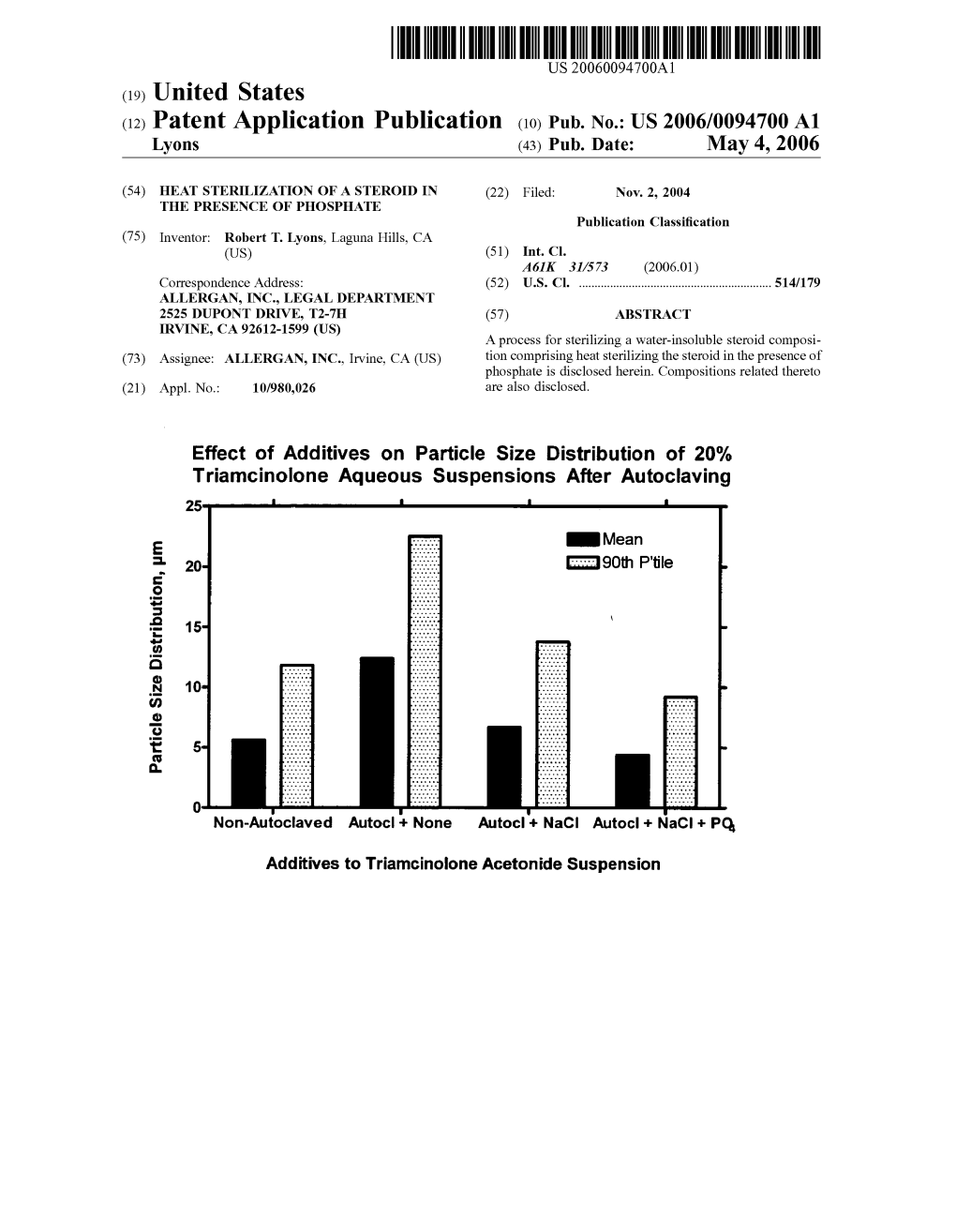
Triamcinolone Aqueous Suspensions After Autoclaving
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Determination of Triamcinolone Acetonide Steroid on Glassy Carbon Electrode by Stripping Voltammetric Methods
Int. J. Electrochem. Sci., 3 (2008) 509-518 International Journal of ELECTROCHEM ICAL SCIENCE www.electrochemsci.org Determination of Triamcinolone Acetonide Steroid on Glassy Carbon Electrode by Stripping Voltammetric Methods C. Vedhi, R. Eswar, H. Gurumallesh Prabu and P. Manisankar* Department of Industrial Chemistry, Alagappa University, Karaikudi –630 003,Tamil Nadu, India *E-mail: [email protected] or [email protected] Received: 9 December 2007 / Accepted: 6 February 2008 / Online published: 20 February 2008 A corticosteroid, triamcinolone acetonide was determined by stripping voltammetric procedure using glassy carbon electrode. Cyclic voltammetric behaviour of steroid was studied in 50% aqueous methanol at acid, neutral and alkaline pH conditions. Influence of pH, sweep rate and steroid concentration were studied. An irreversible and adsorption-controlled well-defined reduction peak was observed in all pH conditions. The reduction peak potential shifted cathodically with change in pH. Controlled potential coulometry revealed two-electron reduction of the α,β-unsaturated carbonyl function present in the steroid. A systematic study of the experimental parameters that affect the differential pulse / square wave stripping voltammetric response was carried out and optimized conditions were arrived at. Under optimum conditions, differential pulse and square wave adsorptive stripping voltammetric procedures were developed for the determination of triamcinolone acetonide steroid at pH 13.0. A calibration plots was derived and the lower limit of determination observed are 0.1 µg/mL from DPSV and 0.01 µg/mL from SWSV. Keywords: Triamcinolone acetonide steroid, Cyclic Voltammetry, Stripping Voltammetry, Glassy carbon electrode. 1. INTRODUCTION Corticosteroids are hormones produced naturally by the adrenal glands, which have many important functions on every organ system. -

Penetration of Synthetic Corticosteroids Into Human Aqueous Humour
Eye (1990) 4, 526--530 Penetration of Synthetic Corticosteroids into Human Aqueous Humour C. N. 1. McGHEE,1.3 D. G. WATSON, 3 1. M. MIDGLEY, 3 M. 1. NOBLE, 2 G. N. DUTTON, z A. I. FERNl Glasgow Summary The penetration of prednisolone acetate (1%) and fluorometholone alcohol (0.1%) into human aqueous humour following topical application was determined using the very sensitive and specific technique of Gas Chromatography with Mass Spec trometry (GCMS). Prednisolone acetate afforded peak mean concentrations of 669.9 ng/ml within two hours and levels of 28.6 ng/ml in aqueous humour were detected almost 24 hours post application. The peak aqueous humour level of flu orometholone was S.lng/ml. The results are compared and contrasted with the absorption of dexamethasone alcohol (0.1%), betamethasone sodium phosphate (0.1 %) and prednisolone sodium phosphate (0.5%) into human aqueous humour. Topical corticosteroid preparations have been prednisolone acetate (1.0%) and fluorometh used widely in ophthalmology since the early alone alcohol (0.1 %) (preliminary results) 1960s and over the last 10 years the choice of into the aqueous humour of patients under preparations has become larger and more going elective cataract surgery. varied. Unfortunately, data on the intraocular penetration of these steroids in humans has SUbjects and Methods not paralleled the expansion in the number of Patients who were scheduled to undergo rou available preparations; indeed until recently, tine cataract surgery were recruited to the estimation of intraocular penetration has study and informed consent was obtained in been reliant upon extrapolation of data from all cases (n=88), Patients with corneal disease animal models (see Watson et ai., 1988, for or inflammatory ocular conditions which bibliography). -

A New Robust Technique for Testing of Glucocorticosteroids in Dogs and Horses Terry E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 A new robust technique for testing of glucocorticosteroids in dogs and horses Terry E. Webster Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Toxicology and Pharmacology Commons Recommended Citation Webster, Terry E., "A new robust technique for testing of glucocorticosteroids in dogs and horses" (2007). Retrospective Theses and Dissertations. 15029. https://lib.dr.iastate.edu/rtd/15029 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A new robust technique for testing of glucocorticosteroids in dogs and horses by Terry E. Webster A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program o f Study Committee: Walter G. Hyde, Major Professor Steve Ensley Thomas Isenhart Iowa State University Ames, Iowa 2007 Copyright © Terry Edward Webster, 2007. All rights reserved UMI Number: 1446027 Copyright 2007 by Webster, Terry E. All rights reserved. UMI Microform 1446027 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii DEDICATION I want to dedicate this project to my wife, Jackie, and my children, Shauna, Luke and Jake for their patience and understanding without which this project would not have been possible. -

Canine Vascular Tissues Are Targets for Androgens, Estrogens, Progestins, and Glucocorticoids
Canine vascular tissues are targets for androgens, estrogens, progestins, and glucocorticoids. K B Horwitz, L D Horwitz J Clin Invest. 1982;69(4):750-758. https://doi.org/10.1172/JCI110513. Research Article Sex differences and steroid hormones are known to influence the vascular system as shown by the different incidence of atherosclerosis in men and premenopausal women, or by the increased risk of cardiovascular diseases in women taking birth control pills or men taking estrogens. However, the mechanisms for these effects in vascular tissues are not known. Since steroid actions in target tissues are mediated by receptors, we have looked for cytoplasmic steroid receptor proteins in vascular tissues of dogs. We find specific saturable receptors, sedimenting at 8S on sucrose density gradients for estrogens (measured with [3H]estradiol +/- unlabeled diethylstilbestrol), androgens (measured with [3H]R1881 +/- unlabeled R1881 and triamcinolone acetonide), and glucocorticoids (measured with [3H]dexamethasone +/- unlabeled dexamethasone); they are absent for progesterone (measured with [3H]R5020 +/- unlabeled R5020 and dihydrotestosterone). Progesterone receptors can, however, be induced by 1-wk treatment of dogs with physiological estradiol concentrations (100 pg/ml serum estrogen), indicating a functional estrogen receptor. Receptor levels range from 20 to 2,000 fmol/mg DNA. They are specific for each hormone; unrelated steroids fail to complete for binding. Low dissociation constants, measured by Scatchard analyses, show that binding is of high affinity. Steroid binding sites are in the media and/or adventitia since they persist when the intima is removed. Compared with the arteries, receptor levels are reduced 80% in inferior venae cavae of […] Find the latest version: https://jci.me/110513/pdf Canine Vascular Tissues Are Targets for Androgens, Estrogens, Progestins, and Glucocorticoids KATHRYN B. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -

Guidelines for Management of Acne
GUIDELINES FOR MANAGEMENT OF ACNE PAGE 1. Classification of Acne 2 2. Medication available at ANMC 2 3. Adjunctive Measures 3 4. Acne treatment by severity or type 3 5. Patient Education 4 Revised by : Jennifer Stroh x3317 Revision Date: 03/01/06 PIC Approval Date: 09/07/06 1 TREATMENT OF ACNE Classification of Acne Severity Papules/Pustules Nodules Comedonal None to few None Mild Few to several None Moderate Several to many Few to several Severe Numerous or extensive Many Medications available at ANMC for acne treatment Topical 1. Bacteriocidal: - Benzoyl peroxide (OTC-non formulary) - antimicrobicidal gel, cream, lotion (2.5, 5, 10%). Apply qhs or bid. May irritate skin resulting in redness and scaling which usually resolves with continued use. 2. Topical antibiotics: - Clindamycin solution. Apply bid - Benzamycin gel* (Erythromycin 3%, Benzoyl peroxide 5%). Apply qhs initially, advance to bid if indicated * Benzamycin gel is second line (restricted per ANMC’s formulary to failed topical clindamycin). 3. Retinoids: - Tretinoin (Retin-A) cream 0.025%, 0.1%. Start with lower strength, applied 2-3 times/wk. Wash skin before bedtime and allow to dry for 30 mins before applying tretinoin. Redness and scaling may occur and acne may worsen over 2-4 weeks. Benzoyl peroxide oxidizes tretinoin, so don’t apply at the same time. Systemic 1. Oral antibiotics. Start with high dose and gradually taper over 2-4 months to lowest maintenance dose required to maintain control. Instruct patients to increase dose with first sign of flare-up. - Tetracycline-250mg, 500mg. Start with 500mg bid, taper monthly to 250 mg bid, then qd or qod. -
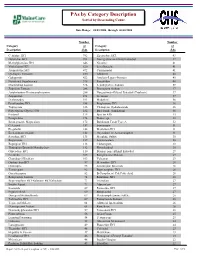
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

OHIO STATE BOARD of PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider
OHIO STATE BOARD OF PHARMACY 77 South High Street, Room 1702; Columbus, OH 43215-6126 -Equal Opportunity Employer and Service Provider- TEL: 614/466-4143 E-MAIL: [email protected] FAX: 614/752-4836 TTY/TDD: Use the Ohio Relay Service: 1-800/750-0750 www.pharmacy.ohio.gov ORDER OF THE STATE BOARD OF PHARMACY (Docket No. D-031110-034) In The Matter Of: CHRISTOPHER KIEL, R.Ph. 8260 Cyrus Lane Sagamore Hills, Ohio 44067 (R.Ph. No. 03-1-12367) INTRODUCTION THE MATTER OF CHRISTOPHER KIEL CAME FOR HEARING ON MAY 4, 2004, BEFORE THE FOLLOWING MEMBERS OF THE BOARD: ROBERT P. GIACALONE, R.Ph. (presiding); DIANE C. ADELMAN, R.Ph.; GREGORY BRAYLOCK, R.Ph.; SUZANNE R. EASTMAN, R.Ph.; ELIZABETH I. GREGG, R.Ph.; LAWRENCE J. KOST, R.Ph.; NATHAN S. LIPSYC, R.Ph.; DOROTHY S. TEATER, PUBLIC MEMBER; AND JAMES E. TURNER, R.Ph. CHRISTOPHER KIEL WAS REPRESENTED BY JOSEPH W. DIEMERT, JR. AND DIANE A. CALTA AND THE STATE OF OHIO WAS REPRESENTED BY SALLY ANN STEUK, ASSISTANT ATTORNEY GENERAL. SUMMARY OF EVIDENCE State’s Witnesses 1. Joann Predina, R.Ph., Ohio State Board of Pharmacy 2. Frederick Lochner, U.S. Food and Drug Administration (FDA) Respondent's Witnesses 1. Christopher Kiel, R.Ph., Respondent State's Exhibits 1K. Copy of Notice of Opportunity For Hearing letter [11-10-03] 1A-1K-E. Procedurals 1K-F. Copy of Amendment Notice [03-03-04] 1G-1K-K. Procedurals 2. Copy of Ohio State Board of Pharmacy Compliance Bulletin 93-002 [09-15-93] 2A. -

Sir, Mistaken Eye Drops and Subsequent Instillation of Superglue
Sir, in susceptible (steroid-responsive) patients. Rimexolone Mistaken eye drops and subsequent instillation of 1 % ophthalmic suspension is a recently developed superglue topical corticosteroid with effective anti-inflammatory z 3 A 60-year-old man presented himself to the casualty properties as well as a reduced risk of increased IOP. , department after accidentally instilling superglue into We present a case report of a patient with markedly his eyes. He had traditionally put eye drops in himself in elevated lOP associated with the use of 1% rimexolone the evening. On this occasion he had mistaken his wife's suspension. fingernail glue for the eye drops as it stood on the bedside cabinet. The bottles were very similar in reduced Case report light, as both were a dropper design for delivery and the same compact size. A 52-year-old woman with a history of toxic epidermal Once the glue, of which the major constituent was necrolysis has been attending our institution since 1992. cyanoacrylate, contacted his eye it caused immense As a result of her condition she developed dry eyes sudden pain causing him to close his eye more. The glue which required punctal occlusion and eye lubricants, then set quickly and thus he presented with a including autologous serum drops. She had marked permanently closed eye. The upper lid was adherent to keratinisation of the tarsal conjunctiva especially in the the cornea, as when the eye movements were tested the left eye, which necessitated mucous membrane grafting. lid moved. Her condition was further complicated by metaplastic He was followed up and after two consultations he eyelashes, which were treated with cryotherapy.