Species-Specific Drought Resilience in Juniper and Fir
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Analyses of Juniperus Species in Turkey and Their Relations with Other Juniperus Based on Cpdna Supervisor: Prof
MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY AYSUN DEMET GÜVENDİREN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY APRIL 2015 Approval of the thesis MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA submitted by AYSUN DEMET GÜVENDİREN in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Department of Biological Sciences, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı Head of the Department, Biological Sciences Prof. Dr. Zeki Kaya Supervisor, Dept. of Biological Sciences METU Examining Committee Members Prof. Dr. Musa Doğan Dept. Biological Sciences, METU Prof. Dr. Zeki Kaya Dept. Biological Sciences, METU Prof.Dr. Hayri Duman Biology Dept., Gazi University Prof. Dr. İrfan Kandemir Biology Dept., Ankara University Assoc. Prof. Dr. Sertaç Önde Dept. Biological Sciences, METU Date: iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Aysun Demet GÜVENDİREN Signature : iv ABSTRACT MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA Güvendiren, Aysun Demet Ph.D., Department of Biological Sciences Supervisor: Prof. -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Relationships Between Wood Formation and Cambium
Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014 Minhui He 1,2, *, Bao Yang 1,Vladimir Shishov 3, 4, Sergio Rossi 5, 6,Achim Bräuning 2,Fredrik Charpentier Ljungqvist 7,8, and Jussi Grießinger 2 1 Key Laboratory of Desert and Desertification, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, 730000 Lanzhou, China; [email protected] (M.H); [email protected] (B.Y.) 2 Institute of Geography, University of Erlangen-Nürnberg, 91058 Erlangen, Germany; [email protected] (A.B.); [email protected] (J.G.) 3 Mathematical Methods and Information Technology Department, Siberian Federal University, L. Prushinskoi street., 2, Krasnoyarsk, 660075, Russia; [email protected] (V.S.) 4 LE STUDIUM Loire Valley Institute for Advanced Studies, 1 rue Dupanloup, 45000 Orléans, France 5 Département des Sciences Fondamentales, Université du Québec à Chicoutimi, Chicoutimi, QC, G7H 2B1, Canada; [email protected] (S.R.) 6 Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China 7 Department of History, Stockholm University, SE-106 91 Stockholm, Sweden; [email protected] (F.C.L.) 8 Bolin Centre for Climate Research, Stockholm University, SE-106 91 Stockholm, Sweden * Correspondence: [email protected]; Tel.: +49-152-3691-7781 1 Figure S1 Averaged mean, minimum and maximum temperatures (lines with filled squares) and precipitation (gray histogram) from January to December at the 20 composite sites during the period 1960–2014. Figure S2 Frequency distributions of the start of the growing season (SOS) in the 2 study region. -
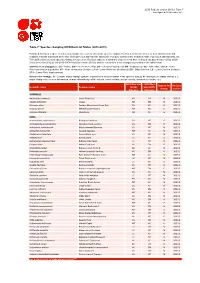
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

A Dual Stable Isotope Approach Unravels Common Climate Signals
geosciences Article A Dual Stable Isotope Approach Unravels Common Climate Signals and Species-Specific Responses to Environmental Change Stored in Multi-Century Tree-Ring Series from the Tibetan Plateau Jussi Grießinger 1,*, Achim Bräuning 1, Gerhard Helle 2, Gerhard Hans Schleser 3, Philipp Hochreuther 1 , Wolfgang Jens-Henrik Meier 1 and Haifeng Zhu 4 1 Institute of Geography, Friedrich-Alexander-University Erlangen-Nürnberg, 91058 Erlangen, Germany; [email protected] (A.B.); [email protected] (P.H.); [email protected] (W.J.-H.M.) 2 GFZ German Research Centre for Geoscience, Section 5.2 Climate Dynamics and Landscape Evolution, 14473 Potsdam, Germany; [email protected] 3 FZJ Research Centre Jülich, Institute of Bio- and Geosciences IBG-3, 52428 Jülich, Germany; [email protected] 4 Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing 100864, China; [email protected] * Correspondence: [email protected]; Tel.: +49-9131-85-22009 Received: 13 February 2019; Accepted: 23 March 2019; Published: 29 March 2019 Abstract: Tree-rings are recorders of environmental signals and are therefore often used to reconstruct past environmental conditions. In this paper, we present four annually resolved, multi-centennial tree-ring isotope series from the southeastern Tibetan plateau. The investigation site, where juniper and spruce trees jointly occur, is one of the highest known tree-stands in the world. Tree ring cellulose oxygen (δ18O) and carbon (δ13C) isotopes were analyzed for a common period of 1685–2007 AD to investigate climate–isotope relationships. Therefore, various climate parameters from a local meteorological station and from the CRU 4.02 dataset were used. -

Identification Key to the Cypress Family (Cupressaceae)1
Feddes Repertorium 116 (2005) 1–2, 96–146 DOI: 10.1002/fedr.200411062 Weinheim, Mai 2005 Ruhr-Universität Bochum, Lehrstuhl für Spezielle Botanik, Bochum C. SCHULZ; P. KNOPF & TH. STÜTZEL Identification key to the Cypress family (Cupressaceae)1 With 11 Figures Summary Zusammenfassung The identification of Cupressaceae taxa, except for Bestimmungsschlüssel für die Familie der Cup- some local and easily distinguishable taxa, is diffi- ressaceae cult even for specialists. One reason for this is the lack of a complete key including all Cupressaceae Die Bestimmung von Cupressaceae-Taxa ist mit taxa, another reason is that diagnoses and descrip- Ausnahme einiger lokaler und leicht bestimmbarer tions are spread over several hundred publications Taxa schwierig, selbst für Spezialisten. Ein Grund, which are sometimes difficult to access. Based on warum es noch keinen vollständigen Bestimmungs- morphological studies of about 3/4 of the species and schlüssel mit allen Cupressaceae-Taxa gibt ist, dass a careful compilation of the most important descrip- die Sippen-Beschreibungen sich auf mehrere hundert tions of Cupressaceae, a first identification key for Publikationen verteilen, welche teilweise schwierig the entire Cypress family (Cupressaceae) could be zu beschaffen sind. Etwa 3/4 der Cupressaceae-Ar- set up. The key comprises any of the 30 genera, 134 ten wurden morphologisch untersucht und die wich- species, 7 subspecies, 38 varieties, one form and thus tigsten Beschreibungen zusammengefasst, daraus all 180 taxa recognized by FARJON (2001). The key wurde dann der erste vollständige Bestimmungs- uses mainly features of adult leaves, female cones schlüssel für Cupressaceae erstellt. Der Bestim- and other characters which are all relatively easy to mungsschlüssel enthält 30 Gattungen, 134 Arten, be used. -

Coniferous Trees - A
FORESTS AND FOREST PLANTS – Vol. II - Coniferous Trees - A. Farjon CONIFEROUS TREES A. Farjon Royal Botanic Gardens, Kew, Richmond, UK Keywords: conifers, species, trees, taxonomy, evolution, palaeobotany, diversity, distribution, ecology, exploitation, conservation Contents 1. Introduction 2. General Overview 3. Species 3.1 Araucariaceae 3.1.1 Agathis australis (Kauri) 3.1.2 Araucaria araucana (Araucaria, Monkey Puzzle Tree) 3.1.3 Araucaria columnaris (Cook Pine) 3.2 Cupressaceae 3.2.1 Callitris columellaris (Cypress Pine) 3.2.2 Cryptomeria japonica (Japanese Cedar, Sugi) 3.2.3 Fitzroya cupressoides (Amerce) 3.2.4 Glyptostrobus pensilis (Water Fir, Shuisong) 3.2.5 Juniperus procera (African Pencil Cedar) 3.2.6 Juniperus tibetica (Tibetan Juniper) 3.2.7 Sequoia sempervirens (Coast Redwood) 3.2.8 Sequoiadendron giganteum (Giant Sequoia, Big Tree) 3.3 Pinaceae 3.3.1 Abies alba (White Fir, Sapin Pectiné, Weisstanne) 3.3.2 Pinus lambertiana (Sugar Pine) 3.3.3 Pinus longaeva (Great Bristlecone Pine) 3.3.4 Pseudotsuga menziesii (Douglas Fir) 3.4 Podocarpaceae 3.4.1 Dacrydium cupressinum (Rimu, Red Pine) 3.4.2 Lagarostrobos franklinii (Huon Pine) 3.4.3 Podocarpus totara (Totara) 3.5 Sciadopityaceae 3.5.1 SciadopitysUNESCO verticillata (Japanese Umbrella– EOLSS Pine) 3.6 Taxaceae 3.6.1 Taxus baccataSAMPLE (Common Yew, European CHAPTERS Yew) Glossary Bibliography Biographical Sketch Summary The diversity of conifers expressed in numbers of species is small in comparison with that of flowering plants. However, the 630 extant species are very diverse, ranging from creeping dwarf shrubs to the tallest trees in the world. They occur on all continents ©Encyclopedia of Life Support Systems (EOLSS) FORESTS AND FOREST PLANTS – Vol. -

Juniperus Bibliography by Workform
Juniperus Bibliography by Workform Record Number: 2110 Author, Analytic: Abdullah-Al-Refai//El-Kateb, H//Stimm, B//Mosandl, R Author Role: Author Affiliation: Article Title: Quality and germination of seeds of Juniperus excelsa M.-Bieb. in the Kalamoun mountains, Syria. Medium Designator: Connective Phrase: Author, Monographic: Author Role: Journal Title: Forstliche Forschungsberichte Munchen Reprint Status: Date of Publication: 2003 Volume Identification: 192 Issue Identification: Page(s): Address/Availability: Lehrstuhl fur Waldbau und Forsteinrichtung, TU Munchen, Germany Location/URL: CODEN: ISSN: 0174-1810 Notes: Abstract: Juniperus excelsa is the main tree species of forest stands in the upper elevations of the Kalamoun mountains in Syria. In this preliminary experiment seeds of juniper from four stands in different elevations (1900, 2100, 2200, 2250 m) were subjected to two pre-treatments with different duration period: warm stratification for three-months followed by 45 days warm stratification followed by 45 days cold stratification, and six months with 90 days warm followed by 90 days cold stratification. In comparison to the other three stands, the stand 2100 above sea level had more vigorous trees from which the seeds were collected. After stratification, seed samples were subjected to a standard germination test according to ISTA regulations. Juniper seeds originating from the Kalamoun mountains showed with 92.5% a high percentage of empty seeds. The better quality of seeds with less empty seeds (87%) were found in the stand which included the more vigorous juniper trees in 2100 m above sea level. Germination of seeds was significantly dependant on the duration period of the warm and cold stratification. -

CUPRESSACEAE.Publish
Flora of China 4: 62–77. 1999. 1 CUPRESSACEAE 柏科 bai ke Fu Liguo (傅立国 Fu Li-kuo)1, Yu Yongfu (于永福)2; Aljos Farjon3 Trees or shrubs evergreen, monoecious or dioecious. Leaves decussate or in whorls of 3, scalelike and then often dimorphic with flattened facial leaves and keeled lateral leaves, or needlelike particularly in juvenile plants, often with an abaxial resin gland. Pollen cones terminal or axillary, solitary, maturing and shed annually; microsporophylls 6–16, decussate or whorled, each bearing (2 or)3–6(–9) pollen sacs; pollen wingless. Seed cones usually terminal, solitary, globose, ovoid, or oblong, dehiscent or indehiscent when mature in 1st or 2nd(or 3rd) year; cone scales developing after ovules originate in bract axils; bracts almost completely enveloped by cone scales, free only at apex; ovules 1–numerous per bract axil, erect; cone scales of mature cones 3–16, flat or peltate, woody, ± leathery, or succulent, 1–20-seeded. Seeds winged or not; wings derived from seed coat. Cotyledons usually 2, rarely 3–6. Germination epigeal. Nineteen genera and ca. 125 species: worldwide; eight genera (one introduced) and 46 species (16 endemic, 13 introduced) in China. In this account, the Cupressaceae is treated sensu stricto, i.e., excluding those taxa that are traditionally classified in Taxodiaceae. A merger of these two families is substantially supported by both morphological and molecular evidence (the Cupressaceae forms a clearcut monophyletic group derived from within the Taxodiaceae). No consistent characters separate them, while the homology of the reproductive organs, so fundamentally different from other conifer families, appears to unite them phylogenetically. -

Juniper Woodlands on the Southern Tibetan Plateau
Between Rear and Leading Edge Juniper woodlands on the southern Tibetan Plateau A high mountain forest-line-ecosystem under environmental change Lars Opgenoorth Between Rear and Leading Edge Juniper woodlands on the southern Tibetan Plateau A high mountain forest-line-ecosystem under environmental change Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) dem Fachbereich Biologie der Philipps-Universität Marburg vorgelegt von Lars Opgenoorth aus Düsseldorf Marburg/Lahn 2009 Vom Fachbereich Biologie der Philipps-Universität Marburg als Dissertation am 11.09.2009 angenommen. Erstgutachterin: Prof. Dr. Birgit Ziegenhagen Zweitgutachter: PD Dr. Karsten Wesche Tag der mündlichen Prüfung am 11.09.2009 List of Papers This thesis is based on the following papers, which are referred to in the text by the Roman numerals I-IV. In addition a research proposal to the Volkswagen foundation is included as part of the outlook. It will be referred to with the roman numeral V. I Miehe, G., Miehe, S., Will, M., Opgenoorth, L., La Duo, Tsering Dorgeh and Liu, J.(2008): An inventory of forest relicts in the pastures of Southern Tibet (Xizang A.R.,China). Journal of Plant Ecology 194: 157–177. II Kaiser, K., Opgenoorth, L., Schoch, W. and Miehe, G. (2009): Charcoal and fossil wood from palaeosols, sediments and artificial structures indicating Late Holocene woodland decline in southern Tibet (China). Quaternary Science Reviews 28: 1539– 1554. III Opgenoorth, L., Vendramin, G., Mao, K., Miehe, G., Miehe, S., Liepelt., S. Liu, J. and Ziegenhagen, B. (2009): Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the Last Glacial Maximum. -

Juniperus Subject Bibliography by Keywords
Juniperus Subject Bibliography by Keywords Juniperus (201) 1. Admasu E.; Thirgood S. J.; Bekele A., and Karen Laurenson M. Spatial ecology of golden jackal in farmland in the Ethiopian Highlands. African Journal of Ecology. 2004; 42(2):144-152. Keywords: Juniperus/ jackal/ Canis aureus/ Ethiopia Abstract: The spatial ecology of golden jackal Canis aureus was studied on farmland adjacent to the Bale Mountains National Park in southern Ethiopia during 1998-2000. Three adult and four subadult jackals were captured in leghold traps and radiotagged. The range size of the adult jackals varied from 7.9 to 48.2 km<sup>2</sup> and the subadults from 24.2 to 64.8 km<sup>2</sup>. These ranges are the largest recorded for this species. Range overlap of the tagged jackals averaged 54%, which, in conjunction with observations of associations between individuals, suggested that all the tagged jackals belonged to one social group. Tagged jackals were observed alone on 87% of occasions despite the extensive overlap in individual ranges. Pairs consisting of a male and female were the most commonly observed group and larger groups were seen on only five occasions. Jackals in this population appeared less gregarious than observed elsewhere. The jackals used all the habitats available to them, particularly at night when they foraged in Artemesia and Hypericum bush and farmland. During the day they were more frequently found in Hagenia and Juniper woodland and their diurnal resting sites were characterized by thick cover. This is the first detailed study of golden jackals in a human- modified landscape in Africa and further demonstrates the flexibility in behaviour and ecology exhibited by this species throughout its range.