Highest Treeline in the Northern Hemisphere Found In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Annotated List of Birds Wintering in the Lhasa River Watershed and Yamzho Yumco, Tibet Autonomous Region, China
FORKTAIL 23 (2007): 1–11 An annotated list of birds wintering in the Lhasa river watershed and Yamzho Yumco, Tibet Autonomous Region, China AARON LANG, MARY ANNE BISHOP and ALEC LE SUEUR The occurrence and distribution of birds in the Lhasa river watershed of Tibet Autonomous Region, People’s Republic of China, is not well documented. Here we report on recent observations of birds made during the winter season (November–March). Combining these observations with earlier records shows that at least 115 species occur in the Lhasa river watershed and adjacent Yamzho Yumco lake during the winter. Of these, at least 88 species appear to occur regularly and 29 species are represented by only a few observations. We recorded 18 species not previously noted during winter. Three species noted from Lhasa in the 1940s, Northern Shoveler Anas clypeata, Solitary Snipe Gallinago solitaria and Red-rumped Swallow Hirundo daurica, were not observed during our study. Black-necked Crane Grus nigricollis (Vulnerable) and Bar-headed Goose Anser indicus are among the more visible species in the agricultural habitats which dominate the valley floors. There is still a great deal to be learned about the winter birds of the region, as evidenced by the number of apparently new records from the last 15 years. INTRODUCTION limited from the late 1940s to the early 1980s. By the late 1980s the first joint ventures with foreign companies were The Lhasa river watershed in Tibet Autonomous Region, initiated and some of the first foreign non-governmental People’s Republic of China, is an important wintering organisations were allowed into Tibet, enabling our own area for a number of migratory and resident bird species. -

Phylogenetic Analyses of Juniperus Species in Turkey and Their Relations with Other Juniperus Based on Cpdna Supervisor: Prof
MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY AYSUN DEMET GÜVENDİREN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY APRIL 2015 Approval of the thesis MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA submitted by AYSUN DEMET GÜVENDİREN in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Department of Biological Sciences, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı Head of the Department, Biological Sciences Prof. Dr. Zeki Kaya Supervisor, Dept. of Biological Sciences METU Examining Committee Members Prof. Dr. Musa Doğan Dept. Biological Sciences, METU Prof. Dr. Zeki Kaya Dept. Biological Sciences, METU Prof.Dr. Hayri Duman Biology Dept., Gazi University Prof. Dr. İrfan Kandemir Biology Dept., Ankara University Assoc. Prof. Dr. Sertaç Önde Dept. Biological Sciences, METU Date: iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Aysun Demet GÜVENDİREN Signature : iv ABSTRACT MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA Güvendiren, Aysun Demet Ph.D., Department of Biological Sciences Supervisor: Prof. -

Reform in Tibet
REFORM IN TIBET AS A SOCIAL MOVEMENT By Luo Jia A thesis submitted in conformity with the requirements for the degree of Master of Education Graduate Department of Sociology & Equity Studies in Education Ontario Institute for Studies in Education University of Toronto © Copyright by Luo Jia (2009) ii REFORM IN TIBET AS A SOCIAL MOVEMENT Master of Education, 2009 Luo Jia Graduate Department of Sociology & Equity Studies in Education Ontario Institute for Studies in Education University of Toronto Abstract Reform as a social process is underresearched in the case of Tibet. This study addresses this gap using Social Movement Theory, which sees social change as a complex process involving various Tibetan social groups and external reformers, the Communist Party of China (CPC). This approach was applied by comparing recruitment and mobilization efforts of several key internal and external reform movements in 20th century Tibetan history. Findings include that internal reform failures can be explained by their narrow social and geographic basis and limited mass appeal. Moreover, initial CPC reforms succeeded through recruitment and mobilization across Tibetan regions and social groupings. Subsequent reforms failed due to decreased attention to recruitment and mass mobilization of Tibetans. A major implication of the study is that understanding social reform in today‟s Tibet requires a SM Theory approach, which currently is lacking among scholars of the Tibetan question and political representatives of both sides. iii Acknowledgements While finishing this work, I thought it is not enough simply to say thanks because the support of many people are behind this research such as family, professors, helpers, and all the people whose work is related to this work. -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

2008 UPRISING in TIBET: CHRONOLOGY and ANALYSIS © 2008, Department of Information and International Relations, CTA First Edition, 1000 Copies ISBN: 978-93-80091-15-0
2008 UPRISING IN TIBET CHRONOLOGY AND ANALYSIS CONTENTS (Full contents here) Foreword List of Abbreviations 2008 Tibet Uprising: A Chronology 2008 Tibet Uprising: An Analysis Introduction Facts and Figures State Response to the Protests Reaction of the International Community Reaction of the Chinese People Causes Behind 2008 Tibet Uprising: Flawed Tibet Policies? Political and Cultural Protests in Tibet: 1950-1996 Conclusion Appendices Maps Glossary of Counties in Tibet 2008 UPRISING IN TIBET CHRONOLOGY AND ANALYSIS UN, EU & Human Rights Desk Department of Information and International Relations Central Tibetan Administration Dharamsala - 176215, HP, INDIA 2010 2008 UPRISING IN TIBET: CHRONOLOGY AND ANALYSIS © 2008, Department of Information and International Relations, CTA First Edition, 1000 copies ISBN: 978-93-80091-15-0 Acknowledgements: Norzin Dolma Editorial Consultants Jane Perkins (Chronology section) JoAnn Dionne (Analysis section) Other Contributions (Chronology section) Gabrielle Lafitte, Rebecca Nowark, Kunsang Dorje, Tsomo, Dhela, Pela, Freeman, Josh, Jean Cover photo courtesy Agence France-Presse (AFP) Published by: UN, EU & Human Rights Desk Department of Information and International Relations (DIIR) Central Tibetan Administration (CTA) Gangchen Kyishong Dharamsala - 176215, HP, INDIA Phone: +91-1892-222457,222510 Fax: +91-1892-224957 Email: [email protected] Website: www.tibet.net; www.tibet.com Printed at: Narthang Press DIIR, CTA Gangchen Kyishong Dharamsala - 176215, HP, INDIA ... for those who lost their lives, for -

Species-Specific Drought Resilience in Juniper and Fir
Ecological Indicators 117 (2020) 106615 Contents lists available at ScienceDirect Ecological Indicators journal homepage: www.elsevier.com/locate/ecolind Species-specific drought resilience in juniper and fir forests in the central Himalayas T ⁎ Ouya Fanga,c, Hongyan Qiua, Qi-Bin Zhanga,b, a State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China b Tibet Agricultural and Animal Husbandry University, Linzhi, China c Swiss Federal Research Institute WSL, Birmensdorf, Switzerland ARTICLE INFO ABSTRACT Keywords: With increased frequency and intensity of drought occurrence in the changing climate, the drought resilience of Resistance forest trees is of widespread interest. Particularly, it is not clear as to how the resilience differs between tree Recovery species and whether or not such resilience changes over time. Understanding tree resilience to drought requires Juniperus tibetica observations not only from recent events but also from the historical past, information of which is usually hardly Abies spectabilis available. Here we defined historical drought based on isotope data and compared drought resilience in Juniperus Oxygen isotope tibetica and Abies spectabilis forest in the central Himalayas in five extreme droughts during the past two cen- Iso/anisohydry fi Tibetan plateau turies. We found that juniper trees had a stronger resistance than r trees in the three extreme droughts in the nineteenth century but this pattern reversed in the two drought events in the twentieth century. The length of response time to droughts and recovery time to pre-drought state were shorter in juniper trees than in fir trees. The proportion of declining trees showed a decreasing trend in fir trees but not in juniper trees. -

Relationships Between Wood Formation and Cambium
Relationships between Wood Formation and Cambium Phenology on the Tibetan Plateau during 1960–2014 Minhui He 1,2, *, Bao Yang 1,Vladimir Shishov 3, 4, Sergio Rossi 5, 6,Achim Bräuning 2,Fredrik Charpentier Ljungqvist 7,8, and Jussi Grießinger 2 1 Key Laboratory of Desert and Desertification, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, 730000 Lanzhou, China; [email protected] (M.H); [email protected] (B.Y.) 2 Institute of Geography, University of Erlangen-Nürnberg, 91058 Erlangen, Germany; [email protected] (A.B.); [email protected] (J.G.) 3 Mathematical Methods and Information Technology Department, Siberian Federal University, L. Prushinskoi street., 2, Krasnoyarsk, 660075, Russia; [email protected] (V.S.) 4 LE STUDIUM Loire Valley Institute for Advanced Studies, 1 rue Dupanloup, 45000 Orléans, France 5 Département des Sciences Fondamentales, Université du Québec à Chicoutimi, Chicoutimi, QC, G7H 2B1, Canada; [email protected] (S.R.) 6 Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China 7 Department of History, Stockholm University, SE-106 91 Stockholm, Sweden; [email protected] (F.C.L.) 8 Bolin Centre for Climate Research, Stockholm University, SE-106 91 Stockholm, Sweden * Correspondence: [email protected]; Tel.: +49-152-3691-7781 1 Figure S1 Averaged mean, minimum and maximum temperatures (lines with filled squares) and precipitation (gray histogram) from January to December at the 20 composite sites during the period 1960–2014. Figure S2 Frequency distributions of the start of the growing season (SOS) in the 2 study region. -
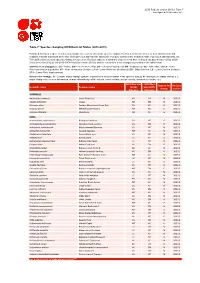
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

The Hidden Monasteries of Tibet
TIBET The Hidden Monasteries of Tibet 19 day itinerary Kathmandu - Lhasa - Drigung Til Monastery Tidrum Nunnery- Reting Monastery - Lake Namtso Gyangtse - Shigatse - Kathmandu Travel dates Starts: Kathmandu - Saturday 3 September 2011 Ends: Kathmandu - Wednesday 21st September 2011 INTRODUCTION For many centuries Tibet remained for westerners a mysterious and forbidden land cut off from the rest of the world by the mighty Himalayas in the south and the Kunlun Mountains to the north. Only a handful of brave and resourceful travellers ever managed to breach the country’s snowy mountain fastness and reach its fabled capital Lhasa. Those that did returned with stories of an exotic and intriguing land of monastic cities and mountain passes, where nomads grazed huge herds of yaks, hermit monks spent years in meditation and pilgrims prostrated themselves over vast distances to achieve their religious goals. In recent years there have been many changes to the Tibetan world but its staggeringly rich, and easily misunderstood, culture remains resilient and continues to fire the imagination of the Western mind. Moreover Tibet is no longer the inaccessible land that it once was, and although travel there is by no means always comfortable, with all its rewards, it is well within the grasp of today’s adventurous traveller. This fascinating journey takes in all the most important sites in Tibet as well as going off-the-beaten-track for a glimpse into a magical Tibet of bygone days. After an unforgettable mountain flight across the Roof of the World we have ample time to explore the sights of Lhasa before setting off in search of the remote hot springs and hidden monasteries that lie in the beautiful countryside to the north of the capital. -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

8 Days Central Lhasa Spiritual
[email protected] +86-28-85593923 8 days Central Lhasa spiritual https://windhorsetour.com/tibet-sightseeing-tour/central-tibet-tour Lhasa Namtso Reting Tidrum Drigung Til Lhasa Explore rural Tibet along a road less traveled. Stay within central Tibet and see the different lifestyles and traditions of the nomads along with traditional villagers. Relax yourself in the unrivaled beauty of holy Namtso Lake. Type Private Duration 8 days Theme Culture and Heritage Trip code WT-109 Price From £ 624 per person Itinerary This tour is a less visited route especially designed for those who want to explore Tibet deeper, experience its rural lifestyles and traditions from a different angle. This clockwise loop tour allows you to see various lifestyles from Jangtang nomads at Namtso to typical villagers within the Nyanang valley, unrivaled beauties of the holy lake and Reting monastery are not only the wonderful part of the trip, but soaking in the natural hotspring at Tidrum nunnery will relax your body before the trip end. Day 01 : Arrival at Lhasa [3,658 m] Upon arrival at Lhasa airport or train station, You will greet by your tour Tibetan tour guide & driver and will transfer to your hotel / hostel in Lhasa, from the airport it is 68km to Lhasa and it takes one and half hour, from the train station it is only 15km and it takes 20 minutes. Afternoon have a good rest to acclimatize the high altitude. Overnight at Lhasa. B=breakfast Day 02 : Lhasa City sightseeing, visit Potola Palace & Jokhang Temple (B) Today is your first day of sightseeing on the high plateau, so we have purposely arranged only to visit Jokhang temple and Potala Palace. -

A Dual Stable Isotope Approach Unravels Common Climate Signals
geosciences Article A Dual Stable Isotope Approach Unravels Common Climate Signals and Species-Specific Responses to Environmental Change Stored in Multi-Century Tree-Ring Series from the Tibetan Plateau Jussi Grießinger 1,*, Achim Bräuning 1, Gerhard Helle 2, Gerhard Hans Schleser 3, Philipp Hochreuther 1 , Wolfgang Jens-Henrik Meier 1 and Haifeng Zhu 4 1 Institute of Geography, Friedrich-Alexander-University Erlangen-Nürnberg, 91058 Erlangen, Germany; [email protected] (A.B.); [email protected] (P.H.); [email protected] (W.J.-H.M.) 2 GFZ German Research Centre for Geoscience, Section 5.2 Climate Dynamics and Landscape Evolution, 14473 Potsdam, Germany; [email protected] 3 FZJ Research Centre Jülich, Institute of Bio- and Geosciences IBG-3, 52428 Jülich, Germany; [email protected] 4 Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing 100864, China; [email protected] * Correspondence: [email protected]; Tel.: +49-9131-85-22009 Received: 13 February 2019; Accepted: 23 March 2019; Published: 29 March 2019 Abstract: Tree-rings are recorders of environmental signals and are therefore often used to reconstruct past environmental conditions. In this paper, we present four annually resolved, multi-centennial tree-ring isotope series from the southeastern Tibetan plateau. The investigation site, where juniper and spruce trees jointly occur, is one of the highest known tree-stands in the world. Tree ring cellulose oxygen (δ18O) and carbon (δ13C) isotopes were analyzed for a common period of 1685–2007 AD to investigate climate–isotope relationships. Therefore, various climate parameters from a local meteorological station and from the CRU 4.02 dataset were used.