Aluminium Sheets, Plates, Coils, Bars Product Specifications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parshwamani Metals
+91-8048554624 Parshwamani Metals https://www.indiamart.com/parshwamanimetals/ Parshwamani Metals is one of the leading manufacturers, supplier and traders of Industrial Metal Tube, Beryllium Product, Shim Sheet, SS Round And Square Bar, Aluminium Products, Aluminum Bronze Products etc. About Us Parshwamani Metals was established in the year 2015 as a professionally managed Manufacturer, Trader and Wholesaler specialized in providing premium grade Copper and Brass Metals Products. Today, we endeavor to revolutionize the industry by fabricating a wide gamut of quality products, which includes Brass Products, Copper Products and Copper Alloy. Our claim to success is hallmarked by the offered quality products that gained us huge recognizance for its high strength, wear and tear resistance, accurate dimensions, flexibility and durable finish. Our products find their wide applications in architectural fittings, hardware and telecommunication. Owing to swift delivery schedules, easy payment modes and overt business practices, we have been successful in earning huge client base. We deal in Jindal Brand. Our efforts are determined with the objective of industrial leadership that equips our team members to manufacture customized products. And, to achieve this, we have developed modernized R&D centers and cutting edge manufacturing facilities. Furthermore, the facility is divided into various functional units like procurement, engineering, production, research & development, quality-testing, warehousing & packaging etc. Our organization is backed -

Downloaded From
Proceedings of The Intl. Conf. on Information, Engineering, Management and Security 2014 [ICIEMS 2014] 144 Effects of Combined Addition of Aluminum Oxide, Fly Ash, Carbon and Yttrium on Density and Hardness of ZA27 Zinc Alloy A. K. Birru a* , R. Manohar Reddy a, B. Srinivas a, K. Balachandan Reddy a and K. Nithin a aDepartment of Mechanical Engineering, Christu Jyothi Institute Of Technology & Science, Jangaon, Warangal, India Abstract: ZA-27 alloy plays a vital role in ZA family of alloys with a high strength and pinnacle applications in manufacturing. The research papers emphasized to enhance hardness and minimize the density of the aforesaid alloy with combined addition of Al 203, fly ash, carbon and yttrium as reinforcements. Hence we observed that with density was gradually decreased at 7% with 5% Al 2O3, 0.15% carbon and 0.01% Yttrium addition. Similarly, further decreased density at 10% with 7.5% Al 2O3, 0.25% carbon and 0.05% Yttrium. However, hardness was initial increased more than 11% with 5% Al 2O3, 0.15% carbon and 0.01% Yttrium. Conversely, hardness was slightly decreased at 5% with 7.5% Al 2O3, 0.25% carbon and 0.05% Yttrium. Keywords: Aluminum oxide; Flyash; Carbon; Yttrium; Density; Hardness. 1. Introduction Zinc alloys with higher aluminium content (25-27 wt. %) obtained by conventional processes of melting and casting, are applied in various fields, particularly in automobile industry, because of their good mechanical, technological and economical properties. Lim Ying Pio et al. [1] conducted the experimentation of LM6 Al- Si alloy on a sand casting of different modulus, the addition level of Al5Ti1B into the melt ranges from 0 wt. -
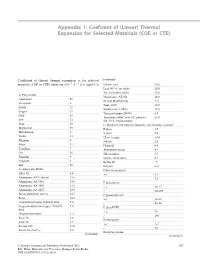
Linear) Thermal Expansion for Selected Materials (COE Or CTE
Appendix 1: Coefcient of (Linear) Thermal Expansion for Selected Materials (COE or CTE) Coefficient of (linear) thermal expansion, α, for selected (continued) − − materials (COE or CTE) (units are ×10 6 °C 1 (i.e. ppm/°C)) Indium–lead 33.0 Lead (95 %) tin solder 28.0 Tin–lead solder 60/40 25.0 A. Pure metals Magnesium, AZ31B 26.0 Aluminium 25 Ni-clad Molybdenum 5–6 Chromium 6 Steel, 1020 12.0 Cobalt 12 Stainless steel (18-8) 17.0 Copper 17 Tungsten/copper (90/10) 6.5 Gold 14 Aluminium MMC with SiC particles 6–14 Iron 12 (80–50 % reinforcement) Lead 29 C. Insulators and substrate materials (for electronic systems)a Magnesium 25 E glass 5.5 Molybdenum 5 S glass 2.6 Nickel 13 Glass–ceramic >3.0 Platinum 9 Silicon 2.6 Silver 19 Diamond 0.9 Tantalum 7 Aluminium nitride 4.5 Tin 20 Silicon nitride 3.7 Titanium 9 Quartz, fused silica 0.5 Tungsten 5 Kevlar 49 –5 Zinc 35 Beryllia 6–9 B. Alloys and MMCs Cubic boron nitride Alloy 42 4.4 x–y 3.7 Aluminium (40 % silicon) 13.5 z 7.2 Aluminium, AA 6061 23.6 E glass/epoxy Aluminium, AA 3003 23.2 x–y 14–17 Aluminium, AA 2017 22.9 z 80–280 Boron aluminium (20 %) 12.7 E glass/polyimide Brass 18.0 x–y 12–16 Copper/invar/copper 20/60/20 thick 5.8 z 40–80 Copper/molybdenum/copper 20/60/20 7.0 E glass/PTFE thick x–y 24 Graphite/aluminium 4–6 z 260 Invar 36 1.6 Kevlar/epoxy Invar 42 4.5 x–y 5–7 Inconel 600 13.0 z 70 Kovar (Fe–Ni–Co) 5.0 (continued) Kevlar/polyimide (continued) © Springer International Publishing Switzerland 2016 557 B.D. -

924 Iaea-Sm-310/ 69P
924 IAEA-SM-310/ 69P RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN M.I. de Vries Netherlands Energy Research Foundation, ECN, Eetten, The Netherlands M.R.. Cundy Joint Research Centre, Patten, The Netherlands 925 RESULTS FROM POST-MORTEM TESTS WITH MATERIAL FROM THE OLD CORE-BOX OF THE HIGH FLUX REACTOR (HFR) AT PETTEN ABSTRACT Results are reported from hardness measurements, tensile tests and fracture mechanics experiments (fatigue crack growth and fracture toughness) on 5154 aluminium specimens, fabricated from remnants of the old HFR core box. The specimen material was exposed to a maximum thermal neutron fluence of 7.5 * 1026n/m2(E < 0.4eV). Test results for this fluence (ratio of the thermal to fast neutron flux density is 1.17) are: hardness 63HR15N, 0.2 - yield strength 525 MPa and total elongation 2.2% strain. Material which was exposed to a lower thermal fluence of 5.6 * 10 n/m , but with a thermal to fast neutron ratio of about 4, shows more radiation hardening : 67HR15N, 0.2 - yield strength 580 MPa and 1.5% total elongation. -5 -3 Fatigue crack growth rates range from 5 * 10 mm/cycle to 10 mm/cycle for AK ranging from 8 to 20 MPa)|m. The most highly exposed (7.5 * 10 n/m ) material shows accelerated fatigue crack growth due to unstable crack extension at AK of about 15 Mpa^m. The lowermost meaningful measure of plane strain fracture toughness is 18 MPa^m. Except for the fracture toughness which is a factor of about 3 higher the results show reasonable agreement with the expected mechanical properties estimated in the "safe end-of-life" assessment of the old HFR vessel. -

Table of Contents
Table of Contents Committees Preface Symposium A: Invited Lectures New Perspectives for Wrought Magnesium Alloys J. Bohlen, D. Letzig and K.U. Kainer 1 Automotive Mg Research and Development in North America J.A. Carpenter, J. Jackman, N.Y. Li, R.J. Osborne, B.R. Powell and P.S. Sklad 11 State of the Art in the Refining and Recycling of Magnesium L.F. Zhang and T. Dupont 25 Research and Development of Processing Technologies for Wrought Magnesium Alloys F.S. Pan, M.B. Yang, Y.L. Ma and G.S. Cole 37 Overview of CAST and Australian Magnesium Research D.H. StJohn 49 Development of Magnesium Alloys with High Performance S. Kamado and Y. Kojima 55 Symposium B: Cast Magnesium Alloys and Foundry Technologies Effects of Microstructure and Partial Melting on Tensile Properties of AZ91 Magnesium Cast Alloy T.P. Zhu, Z.W. Chen and W. Gao 65 Effect of Heat Treatment on the Microstructure and Creep Behavior of Mg-Sn-Ca Alloys T.A. Leil, Y.D. Huang, H. Dieringa, N. Hort, K.U. Kainer, J. Buršík, Y. Jirásková and K.P. Rao 69 Creep Behaviour and Microstructure of Magnesium Die Cast Alloys AZ91 and AE42 K. Wei, L.Y. Wei and R. Warren 73 Growth Rate of Small Fatigue Cracks of Cast Magnesium Alloy at Different Conditions X.S. Wang and J.H. Fan 77 A Cyclic Stress-Strain Constitutive Model for Polycrystalline Magnesium Alloy and its Application X.G. Zeng, Q.Y. Wang, J.H. Fan, Z.H. Gao and X.H. Peng 81 Dynamic Stress-Strain Behavior of AZ91 Alloy at High-Strain Rate G.Y. -

Machining of Aluminum and Aluminum Alloys / 763
ASM Handbook, Volume 16: Machining Copyright © 1989 ASM International® ASM Handbook Committee, p 761-804 All rights reserved. DOI: 10.1361/asmhba0002184 www.asminternational.org MachJning of Aluminum and AlumJnum Alloys ALUMINUM ALLOYS can be ma- -r.. _ . lul Tools with small rake angles can normally chined rapidly and economically. Because be used with little danger of burring the part ," ,' ,,'7.,','_ ' , '~: £,~ " ~ ! f / "' " of their complex metallurgical structure, or of developing buildup on the cutting their machining characteristics are superior ,, A edges of tools. Alloys having silicon as the to those of pure aluminum. major alloying element require tools with The microconstituents present in alumi- larger rake angles, and they are more eco- num alloys have important effects on ma- nomically machined at lower speeds and chining characteristics. Nonabrasive con- feeds. stituents have a beneficial effect, and ,o IIR Wrought Alloys. Most wrought alumi- insoluble abrasive constituents exert a det- num alloys have excellent machining char- rimental effect on tool life and surface qual- acteristics; several are well suited to multi- ity. Constituents that are insoluble but soft B pie-operation machining. A thorough and nonabrasive are beneficial because they e,,{' , understanding of tool designs and machin- assist in chip breakage; such constituents s,~ ,.t ing practices is essential for full utilization are purposely added in formulating high- of the free-machining qualities of aluminum strength free-cutting alloys for processing in alloys. high-speed automatic bar and chucking ma- Strain-hardenable alloys (including chines. " ~ ~p /"~ commercially pure aluminum) contain no In general, the softer ailoys~and, to a alloying elements that would render them lesser extent, some of the harder al- c • o c hardenable by solution heat treatment and ,p loys--are likely to form a built-up edge on precipitation, but they can be strengthened the cutting lip of the tool. -

Aluminium Alloys Chemical Composition Pdf
Aluminium alloys chemical composition pdf Continue Alloy in which aluminum is the predominant lye frame of aluminum welded aluminium alloy, manufactured in 1990. Aluminum alloys (or aluminium alloys; see spelling differences) are alloys in which aluminium (Al) is the predominant metal. Typical alloy elements are copper, magnesium, manganese, silicon, tin and zinc. There are two main classifications, namely casting alloys and forged alloys, both further subdivided into heat-treatable and heat-free categories. Approximately 85% of aluminium is used for forged products, e.g. laminated plates, foils and extrusions. Aluminum cast alloys produce cost-effective products due to their low melting point, although they generally have lower tensile strength than forged alloys. The most important cast aluminium alloy system is Al–Si, where high silicon levels (4.0–13%) contributes to giving good casting features. Aluminum alloys are widely used in engineering structures and components where a low weight or corrosion resistance is required. [1] Alloys composed mostly of aluminium have been very important in aerospace production since the introduction of metal leather aircraft. Aluminum-magnesium alloys are both lighter than other aluminium alloys and much less flammable than other alloys containing a very high percentage of magnesium. [2] Aluminum alloy surfaces will develop a white layer, protective of aluminum oxide, if not protected by proper anodization and/or dyeing procedures. In a wet environment, galvanic corrosion can occur when an aluminum alloy is placed in electrical contact with other metals with a more positive corrosion potential than aluminum, and an electrolyte is present that allows the exchange of ions. -

Aluminum Alloy Weldability: Identification of Weld Solidification Cracking Mechanisms Through Novel Experimental Technique and Model Development
Dipl.-Ing. Nicolas Coniglio Aluminum Alloy Weldability: Identifi cation of Weld Solidifi cation Cracking Mechanisms through Novel Experimental Technique and Model Development BAM-Dissertationsreihe • Band 40 Berlin 2008 Die vorliegende Arbeit entstand an der BAM Bundesanstalt für Materialforschung und -prüfung. Impressum Aluminum Alloy Weldability: Identifi cation of Weld Solidifi cation Cracking Mechanisms through Novel Experimental Technique and Model Development 2008 Herausgeber: BAM Bundesanstalt für Materialforschung und -prüfung Unter den Eichen 87 12205 Berlin Telefon: +49 30 8104-0 Telefax: +49 30 8112029 E-Mail: [email protected] Internet: www.bam.de Copyright © 2008 by BAM Bundesanstalt für Materialforschung und -prüfung Layout: BAM-Arbeitsgruppe Z.64 ISSN 1613-4249 ISBN 978-3-9812354-3-2 Aluminum Alloy Weldability: Identification of Weld Solidification Cracking Mechanisms through Novel Experimental Technique and Model Development Dissertation zur Erlangung des akademischen Grades Doktor-Ingenieur (Dr.-Ing.) genehmigt durch die Fakultät für Maschinenbau der Otto-von-Guericke-Universität Madgeburg am 02.06.08 vorgelegte Dissertation von Dipl.-Ing. Nicolas Coniglio Thesis Committee: Prof. Dr.-Ing. A. Bertram Prof. Dr.-Ing. T. Böllinghaus Prof. C.E. Cross Prof. S. Marya Date of Examination: 23 October 2008 Abstract Abstract The objective of the present thesis is to make advancements in understanding solidification crack formation in aluminum welds, by investigating in particular the aluminum 6060/4043 system. Alloy 6060 is typical of a family of Al-Mg-Si extrusion alloys, which are considered weldable only when using an appropriate filler alloy such as 4043 (Al-5Si). The effect of 4043 filler dilution (i.e. weld metal silicon content) on cracking sensitivity and solidification path of Alloy 6060 welds are investigated. -

International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys
International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys 1525 Wilson Boulevard, Arlington, VA 22209 www.aluminum.org With Support for On-line Access From: Aluminum Extruders Council Australian Aluminium Council Ltd. European Aluminium Association Japan Aluminium Association Alro S.A, R omania Revised: January 2015 Supersedes: February 2009 © Copyright 2015, The Aluminum Association, Inc. Unauthorized reproduction and sale by photocopy or any other method is illegal . Use of the Information The Aluminum Association has used its best efforts in compiling the information contained in this publication. Although the Association believes that its compilation procedures are reliable, it does not warrant, either expressly or impliedly, the accuracy or completeness of this information. The Aluminum Association assumes no responsibility or liability for the use of the information herein. All Aluminum Association published standards, data, specifications and other material are reviewed at least every five years and revised, reaffirmed or withdrawn. Users are advised to contact The Aluminum Association to ascertain whether the information in this publication has been superseded in the interim between publication and proposed use. CONTENTS Page FOREWORD ........................................................................................................... i SIGNATORIES TO THE DECLARATION OF ACCORD ..................................... ii-iii REGISTERED DESIGNATIONS AND CHEMICAL COMPOSITION -

Arc Welding of Nonferrous Metals Arc Welding of Nonferrous Metals
Arc Welding of Nonferrous Metals Arc Welding of Nonferrous Metals Published by The Arc Welding of Nonferrous Metals KOBE STEEL, LTD. is a textbook for providing information to assist welding personnel study © 2015 by KOBE STEEL, LTD. the arc welding technologies commonly 5-912, Kita-Shinagawa, Shinagawa-Ku, applied in the equipment made from Tokyo, 141-8688 Japan aluminum, aluminum alloys, copper, copper alloys, nickel, and nickel alloys. All rights reserved. No part of this book may be reproduced, Reasonable care is taken in any form or by any means, without in the compilation and publication of permission in writing from this textbook to insure authenticity of the publisher the contents. No representation or warranty is made as to the accuracy or reliability of this information. Forewords Nonferrous metals are non-iron-based metals such as aluminum and aluminum alloys, copper and copper alloys, nickel and nickel alloys, titanium and titanium alloys, and magnesium and magnesium alloys. Today, nonferrous metals are used in various welding constructions for diverse industrial applications. However, their weldability is quite different from that of steel, due to specific physical and metallurgical characteristics. Therefore, the welding procedure for nonferrous metals should be thoroughly examined taking into account the inherent characteristics of the particular nonferrous metal to be welded, in order to get sound weldments. This textbook focuses on the arc welding of aluminum, aluminum alloys, copper, copper alloys, nickel, and nickel alloys that are used more extensively over other nonferrous metals for industrial applications. This textbook consists of three chapters: Chapter 1: Arc Welding of Aluminum and Aluminum Alloys Chapter 2: Arc Welding of Copper and Copper Alloys Chapter 3: Arc Welding of Nickel and Nickel Alloys iii Chapter 1 Arc Welding of Aluminum and Aluminum Alloys Contents Introduction 2 1. -

Aluminium ALUMINIUM the RIGHT MATERIAL for OUR ENVIRONMENT
Aluminium ALUMINIUM THE RIGHT MATERIAL FOR OUR ENVIRONMENT ycle ec R M e l t The Life Cycle of Aluminium U s e te ica Fabr A complete life cycle material for the Consumer, Industry and the Environment. To ensure a high quality of life, the materials that we as consumers and manufacturers use, should meet not only technical performance standards, but have a long service life, be useable in a greater number of applications and be environmentally friendly. Once their service is complete, they should be 100% recyclable, thereby completing the life cycle to be used once again. Aluminium is such a material. ISO 9001 Lic QEC 3491 SAI Global Page 2 Welcome to Austral Wright Metals is the result of the merging, on 1st December 1997, of two long established well respected Australian owned metal distribution companies. Austral Bronze Crane Copper Limited (the metal distribution division of the Crane Group) and Wright and Company Pty Limited. This brought together Australia’s leaders in the distribution of: Copper, brass and bronze – sheet, coil, bar, rod, extrusions and tube. Stainless steel – sheet, coil, plate, bar, rod tube and fittings. Aluminum – sheet, coil, plate and tread plate. High Performance Alloys – including nickel based alloys, welding consumables and high technology metals. Austral Bronze Crane Copper was incorporated in 1914 to manufacture non ferrous sheet, coil and extruded product. The business was restructured in 1990 to clearly focus on the distribution of non ferrous and specialty metals. Incorporated in 1913, Wright and Company concentrated its efforts on the distribution of stainless steel and non ferrous alloys through its Australia wide warehouse network. -

Aluminum Foundry Products
ASM Handbook, Volume 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials Copyright © 1990 ASM International® ASM Handbook Committee, p 123-151 All rights reserved. DOI: 10.1361/asmhba0001061 www.asminternational.org Aluminum Foundry Products Revised by A. Kearney, Avery Kearney & Company Elwin L. Rooy, Aluminum Company of America ALUMINUM CASTING ALLOYS are wrought alloys. Aluminum casting alloys cast aluminum alloys are grouped according the most versatile of all common foundry must contain, in addition to strengthening to composition limits registered with the alloys and generally have the highest cast- elements, sufficient amounts of eutectic- Aluminum Association (see Table 3 in the ability ratings. As casting materials, alumi- forming elements (usually silicon) in order article "Alloy and Temper Designation Sys- num alloys have the following favorable to have adequate fluidity to feed the shrink- tems for Aluminum and Aluminum Al- characteristics: age that occurs in all but the simplest cast- loys"). Comprehensive listings are also • Good fluidity for filling thin sections ings. maintained by general procurement specifi- The phase behavior of aluminum-silicon • Low melting point relative to those re- cations issued through government agencies compositions (Fig. 1) provides a simple quired for many other metals (federal, military, and so on) and by techni- • Rapid heat transfer from the molten alu- eutectic-forming system, which makes pos- cal societies such as the American Society sible the commercial viability of most high- minum to the mold, providing shorter for Testing and Materials and the Society of casting cycles volume aluminum casting. Silicon contents, Automotive Engineers (see Table 1 for ex- • Hydrogen is the only gas with apprecia- ranging from about 4% to the eutectic level amples).