Aluminum MIG Wire from Lincoln Electric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded From
Proceedings of The Intl. Conf. on Information, Engineering, Management and Security 2014 [ICIEMS 2014] 144 Effects of Combined Addition of Aluminum Oxide, Fly Ash, Carbon and Yttrium on Density and Hardness of ZA27 Zinc Alloy A. K. Birru a* , R. Manohar Reddy a, B. Srinivas a, K. Balachandan Reddy a and K. Nithin a aDepartment of Mechanical Engineering, Christu Jyothi Institute Of Technology & Science, Jangaon, Warangal, India Abstract: ZA-27 alloy plays a vital role in ZA family of alloys with a high strength and pinnacle applications in manufacturing. The research papers emphasized to enhance hardness and minimize the density of the aforesaid alloy with combined addition of Al 203, fly ash, carbon and yttrium as reinforcements. Hence we observed that with density was gradually decreased at 7% with 5% Al 2O3, 0.15% carbon and 0.01% Yttrium addition. Similarly, further decreased density at 10% with 7.5% Al 2O3, 0.25% carbon and 0.05% Yttrium. However, hardness was initial increased more than 11% with 5% Al 2O3, 0.15% carbon and 0.01% Yttrium. Conversely, hardness was slightly decreased at 5% with 7.5% Al 2O3, 0.25% carbon and 0.05% Yttrium. Keywords: Aluminum oxide; Flyash; Carbon; Yttrium; Density; Hardness. 1. Introduction Zinc alloys with higher aluminium content (25-27 wt. %) obtained by conventional processes of melting and casting, are applied in various fields, particularly in automobile industry, because of their good mechanical, technological and economical properties. Lim Ying Pio et al. [1] conducted the experimentation of LM6 Al- Si alloy on a sand casting of different modulus, the addition level of Al5Ti1B into the melt ranges from 0 wt. -

Table of Contents
Table of Contents Committees Preface Symposium A: Invited Lectures New Perspectives for Wrought Magnesium Alloys J. Bohlen, D. Letzig and K.U. Kainer 1 Automotive Mg Research and Development in North America J.A. Carpenter, J. Jackman, N.Y. Li, R.J. Osborne, B.R. Powell and P.S. Sklad 11 State of the Art in the Refining and Recycling of Magnesium L.F. Zhang and T. Dupont 25 Research and Development of Processing Technologies for Wrought Magnesium Alloys F.S. Pan, M.B. Yang, Y.L. Ma and G.S. Cole 37 Overview of CAST and Australian Magnesium Research D.H. StJohn 49 Development of Magnesium Alloys with High Performance S. Kamado and Y. Kojima 55 Symposium B: Cast Magnesium Alloys and Foundry Technologies Effects of Microstructure and Partial Melting on Tensile Properties of AZ91 Magnesium Cast Alloy T.P. Zhu, Z.W. Chen and W. Gao 65 Effect of Heat Treatment on the Microstructure and Creep Behavior of Mg-Sn-Ca Alloys T.A. Leil, Y.D. Huang, H. Dieringa, N. Hort, K.U. Kainer, J. Buršík, Y. Jirásková and K.P. Rao 69 Creep Behaviour and Microstructure of Magnesium Die Cast Alloys AZ91 and AE42 K. Wei, L.Y. Wei and R. Warren 73 Growth Rate of Small Fatigue Cracks of Cast Magnesium Alloy at Different Conditions X.S. Wang and J.H. Fan 77 A Cyclic Stress-Strain Constitutive Model for Polycrystalline Magnesium Alloy and its Application X.G. Zeng, Q.Y. Wang, J.H. Fan, Z.H. Gao and X.H. Peng 81 Dynamic Stress-Strain Behavior of AZ91 Alloy at High-Strain Rate G.Y. -

International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys
International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys 1525 Wilson Boulevard, Arlington, VA 22209 www.aluminum.org With Support for On-line Access From: Aluminum Extruders Council Australian Aluminium Council Ltd. European Aluminium Association Japan Aluminium Association Alro S.A, R omania Revised: January 2015 Supersedes: February 2009 © Copyright 2015, The Aluminum Association, Inc. Unauthorized reproduction and sale by photocopy or any other method is illegal . Use of the Information The Aluminum Association has used its best efforts in compiling the information contained in this publication. Although the Association believes that its compilation procedures are reliable, it does not warrant, either expressly or impliedly, the accuracy or completeness of this information. The Aluminum Association assumes no responsibility or liability for the use of the information herein. All Aluminum Association published standards, data, specifications and other material are reviewed at least every five years and revised, reaffirmed or withdrawn. Users are advised to contact The Aluminum Association to ascertain whether the information in this publication has been superseded in the interim between publication and proposed use. CONTENTS Page FOREWORD ........................................................................................................... i SIGNATORIES TO THE DECLARATION OF ACCORD ..................................... ii-iii REGISTERED DESIGNATIONS AND CHEMICAL COMPOSITION -

Aluminum Foundry Products
ASM Handbook, Volume 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials Copyright © 1990 ASM International® ASM Handbook Committee, p 123-151 All rights reserved. DOI: 10.1361/asmhba0001061 www.asminternational.org Aluminum Foundry Products Revised by A. Kearney, Avery Kearney & Company Elwin L. Rooy, Aluminum Company of America ALUMINUM CASTING ALLOYS are wrought alloys. Aluminum casting alloys cast aluminum alloys are grouped according the most versatile of all common foundry must contain, in addition to strengthening to composition limits registered with the alloys and generally have the highest cast- elements, sufficient amounts of eutectic- Aluminum Association (see Table 3 in the ability ratings. As casting materials, alumi- forming elements (usually silicon) in order article "Alloy and Temper Designation Sys- num alloys have the following favorable to have adequate fluidity to feed the shrink- tems for Aluminum and Aluminum Al- characteristics: age that occurs in all but the simplest cast- loys"). Comprehensive listings are also • Good fluidity for filling thin sections ings. maintained by general procurement specifi- The phase behavior of aluminum-silicon • Low melting point relative to those re- cations issued through government agencies compositions (Fig. 1) provides a simple quired for many other metals (federal, military, and so on) and by techni- • Rapid heat transfer from the molten alu- eutectic-forming system, which makes pos- cal societies such as the American Society sible the commercial viability of most high- minum to the mold, providing shorter for Testing and Materials and the Society of casting cycles volume aluminum casting. Silicon contents, Automotive Engineers (see Table 1 for ex- • Hydrogen is the only gas with apprecia- ranging from about 4% to the eutectic level amples). -

Aluminum and Aluminum Alloys
Alloying: Understanding the Basics Copyright © 2001 ASM International® J.R. Davis, p351-416 All rights reserved. DOI:10.1361/autb2001p351 www.asminternational.org Aluminum and Aluminum Alloys Introduction and Overview General Characteristics. The unique combinations of properties provided by aluminum and its alloys make aluminum one of the most ver- satile, economical, and attractive metallic materials for a broad range of uses—from soft, highly ductile wrapping foil to the most demanding engi- neering applications. Aluminum alloys are second only to steels in use as structural metals. Aluminum has a density of only 2.7 g/cm3, approximately one-third as much as steel (7.83 g/cm3). One cubic foot of steel weighs about 490 lb; a cubic foot of aluminum, only about 170 lb. Such light weight, coupled with the high strength of some aluminum alloys (exceeding that of struc- tural steel), permits design and construction of strong, lightweight structures that are particularly advantageous for anything that moves—space vehi- cles and aircraft as well as all types of land- and water-borne vehicles. Aluminum resists the kind of progressive oxidization that causes steel to rust away. The exposed surface of aluminum combines with oxygen to form an inert aluminum oxide film only a few ten-millionths of an inch thick, which blocks further oxidation. And, unlike iron rust, the aluminum oxide film does not flake off to expose a fresh surface to further oxidation. If the protective layer of aluminum is scratched, it will instantly reseal itself. The thin oxide layer itself clings tightly to the metal and is colorless and transparent—invisible to the naked eye. -

Ferroelectric and Ferromagnetic Alloy Clusters in Molecular Beams Shuangye
Ferroelectric and Ferromagnetic Alloy Clusters in Molecular Beams A Thesis Presented to The Academic Faculty by Shuangye Yin In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy School of Physics Georgia Institute of Technology August 2006 Ferroelectric and Ferromagnetic Alloy Clusters in Molecular Beams Approved by: Professor Walter A. de Heer, Advisor Professor Brian Kennedy School of Physics School of Physics Georgia Institute of Technology Georgia Institute of Technology Professor Edward H. Conrad Professor Robert L. Whetten School of Physics Department of Chemistry and Biochem- Georgia Institute of Technology istry Georgia Institute of Technology Professor Phillip First School of Physics Georgia Institute of Technology Date Approved: May 9, 2006 To my parents and to my wife. iii ACKNOWLEDGEMENTS I first want to thank my advisor Dr. Walter A. de Heer. In the last five years, he gives me intensive training as a physicist. His insight in physics, research styles and diligence all provide good examples for my future career. I will benefit from his various suggestions in every detail of the scientific activities. I also enjoy so many years of collabrations with Xiaoshan Xu and Ramiro Moro. We finished incredibly difficult jobs together. I am glad that Anthony Liang and John Bowlan joined this lab and made significant contributions. I appreciate the time my committee members spent on my thesis, as well as the sugges- tions and question about this work. I want to thank Dr. Kennedy for valuable discussions and recommendations. I also benefit from talks with Dr. Whetten, Dr. First, Dr. Conrad, Dr. Berger, Dr. -
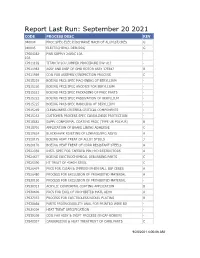
Process Specifications
Report Last Run: September 20 2021 CODE PROCESS DESC REV 940004 PROC SPEC-ELEC DISCHARGE MACH OF ALUM,ST,CRES G 940005 ELECTRCHEMCL DEBURRG G CPS04082- PWR SUPPLY 24VDC 10A - 10A CPS11192 TITAN IV LCU JUMPER PROCEDURE INV U13 - CPS11983 ASSY AND INSP OF GMD ROTOR ASSY 17E647 B CPS11989 COIL FAB ASSEMBLY/INSPECTION PROCESS C CPS15219 BOEING PRCS SPEC MACHINING OF BERYLLIUM - CPS15220 BOEING PRCS SPEC ANODIZE FOR BERYLLIUM - CPS15221 BOEING PRCS SPEC PACKAGING OF PREC PARTS - CPS15222 BOEING PRCS SPEC PASSIVATION OF BERYLLIUM - CPS15225 BOEING PRCS SPEC HANDLING OF BERYLLIUM - CPS15249 CLEANLINESS CRITERIA CRITICAL COMPONENTS - CPS15253 CUSTOMER PROCESS SPEC CLEANLINESS PROTECTION - CPS18682 SUPPL CONFORMAL COATING PROC (TYPE UR POLYUR) B CPS18701 APPLICATION OF BRAKE LINING ADHESIVE C CPS19824 BLACKHAWK RIVETING OF LINKAGE/BRG ASSYS A CPS20175 BOEING HEAT TREAT OF ALLOY STEELS J CPS20176 BOEING HEAT TREAT OF CORR RESISTANT STEELS A CPS22356 INSTL SPEC FOR TAPERED PIN HYD RESTRICTORS A CPS24677 BOEING ELECTROCHEMICAL DEBURRING PARTS C CPS25390 HT TREAT OF 4340M STEEL C CPS26474 PRCS FOR CLEAN & IMPREGN PHEN BALL SEP CERES A CPS26480 PROCESS FOR EXCLUSION OF PROHIBITED MATERIAL A CPS30150 PROCESS FOR EXCLUSION OF PROHIBITED MATERIAL - CPS30313 ACRYLIC CONFORMAL COATING APPLICATION B CPS30606 PRCS FOR EXCL OF PROHIBITED MATL AEHV A CPS37037 PROCESS FOR ELECTROLESS NICKEL PLATING B CPS38888 PARTS PRODUCEABILITY ANAL FOR PRINTED WIRE BD - CPS39204 HEAT TREAT SPECIFICATION - CPS39209 COIL FAB ASSY & INSPT PROCESS (ENCAP BOBBIN) - CPS40357 CARBURIZING -

Terminology Charts Used in Euratom's Nuclear Documentation System
<% 3* m EUR SOO e (SECOND EDITION, PART TWO) EUROPEAN ATOMIC ENERGY COMMUNITY EURATOM EURATOMTHESAURUS PART II TERMINOLOGY CHARTS USED IN EURATOM'S NUCLEAR DOCUMENTATION SYSTEM : M m 'φ -&i-}*0 t ,tn,t.f I» : i&i M Dissemination of Information m Center for Information and Documentation CID Ufi«2r «WA m . Pili * Ali UIA'" pp?. »♦"■'Ι' " l»lt m: mk m- piu** m'ti *ΓΛ PIA This document was prepared under the sponsorship of the iilli of the European Communities. :|ÍBÍ|Í|ÍBKÍÍ,:''!:?; to the accuracy, completeness, or usefulness of the information con jji?J|ijjj|j : IJ;Pipili. tained in this document, or that the use of any information, apparatus, * H" ^ ''¡ti method, or process disclosed in this document may not infringe ¡J^flrøl^j'wiPHii'w'ii" kJ privately „„ed ri8ht,; or #|^1|1Α «tifi Assume any liability with respect to the use of, or for damages resulting _ j·^ : from the use of any information, apparatus, method or process inJiiSijiHj M ¡ m disclosed in this document. ΜΙ<\\',Λ?;> mr Mf«í ţmwm . ,. .{¡¿» ÎAÎ.Ï .»rf U t~U ui'. tOJ. at the price of FF 30, FB 300,— DM 24,— Lit. 3740 Fl. 21,60 $ 6 — m When ordering, please quote the EUR number and the title, ίίη^ί.« Γ which are indicated on the cover of each report. ÍÍRAHÜÍ fm Printed by Guyot Brussels, December w II EUR SOO e (SECOND EDITION, PART TWO) EUROPEAN ATOMIC ENERGY COMMUNITY - EURATOM EURATOM-THESAURUS PART II TERMINOLOGY CHARTS USED IN EURATOM'S NUCLEAR DOCUMENTATION SYSTEM 1967 PARtEriEflT EUROPErn Dissemination of Information « 031897 C-yvvi, Center for Information and -

Aluminium Sheets, Plates, Coils, Bars Product Specifications
Introduction : Aluminium is found primarily in bauxite ore .Pure aluminium is soft, silvery , ductile of the poor metal group of chemical elements ,which is corrosion resistant, light weight and high electrical conductivity . It has the symbol Al and atomic number 13. The metal is used in many industries to manufacture a large variety of products and is very important to the world economy. Structural components made from aluminium and its alloys are vital to the aerospace industry and very important in other areas of transportation and building. It is widely used for foil and conductor cables, but alloying with other elements is necessary to provide the higher strengths needed for other applications. Properties : 1.Aluminium is a soft, lightweight metal with normally a dull silvery appearance caused by a thin layer of oxidation that forms quickly when the metal is exposed to air. 2.Aluminium oxide has a higher melting point than pure aluminium. Aluminium is nontoxic (as the metal), nonmagnetic, and nonsparking. 3.It has a tensile strength of about 49 megapascals (MPa) in a pure state and 400 MPa as an alloy. 4.Aluminium is about one-third as dense as steel or copper; it is malleable, ductile, and easily machinable and castable. 5.It has excellent corrosion resistance and durability because of the protective oxide layer. 6.Aluminium mirror finish has the highest reflectance of any metal in the 200-400 nm (UV) and the 3000-10000 nm (far IR) regions, while in the 400-700 nm visible range it is slightly outdone by silver and in the 700-3000 (near IR) by silver, gold, and copper.