Water Quality Monitoring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EIA Final Report (PDF)
YB299A ENVIRONMENTAL IMPACT ASSESSMENT REPORT FOR MODIFICATIONS TO THE PETITCODIAC RIVER CAUSEWAY Submitted to: New Brunswick Department of Supply and Services Fredericton, New Brunswick Submitted by: AMEC Earth & Environmental, A Division of AMEC Americas Limited Fredericton, New Brunswick September 30, 2005 TE23520.4 NB Department of Supply and Services EIA Report Modifications to the Petitcodiac River Causeway September 2005 EXECUTIVE SUMMARY Background This document is the Environmental Impact Assessment (EIA) Report for the Modifications to the Petitcodiac River Causeway Project. The Petitcodiac River causeway (“causeway”) is a gated dam structure with an installed vertical slot fishway that was built across the Petitcodiac River between the City of Moncton and the Town of Riverview. Figure 1 shows the location of the Petitcodiac River and Figure 2 shows an aerial view of the causeway. Completed in 1968, the causeway was intended to create a second transportation link between the two communities, offer flood protection for farmland between the causeway and the head of tide at Salisbury, and create a freshwater headpond with potential for recreation and as an industrial water source. A bridge would have achieved the transportation objective, but not the other benefits. As early as 1961, it was recognized by Fisheries and Oceans Canada (“DFO”) that fish passage would be an issue if a causeway was built across the Petitcodiac River. Consequently, DFO required that a fishway be included in the structure. However, the construction of the causeway with the fishway resulted in fish passage issues from the outset. The fishway proved ineffective for all fish species that require passage for life cycle purposes, including the Inner Bay of Fundy Atlantic salmon that is now also protected under the Species at Risk Act because of declining numbers. -

All Donations Recognized in the “Friends for Life” Donor Recognition Program Are Cumulative Over the Lifetime of the Donor Beginning January 1, 1990
All donations recognized in the “Friends for Life” donor recognition program are cumulative over the lifetime of the donor beginning January 1, 1990. Tous les dons reconnus dans le cadre du programme de reconnaissance des donateurs Amis pour la vie sont cumulatifs jusqu’au décès du donateur à compter du 1er janvier 1990. Dream Builders /Bâtisseur de rêves $150,000 + President's Circle / Cercle du Président / Cercle de la Présidente $100,000 – $149,999 Benefactor / Bienfaiteur / Bienfaitrice $50,000 – $99,999 Partner / Partenaire $25,000 – $49,999 Companion / Compagnon / Compagne $10,000 – $24,999 Associate gold / Assococié or $5,000 – $9,999 Associate silver / Assococié argent $1,000 – $4,999 Aliant Dream Builder Assomption Vie / Assumption Life Dream Builder C-103 / XL96 Dream Builder Estate of Ruth and Murray Caines Dream Builder Canadian Breast Cancer Foundation-Atlantic Chapter Dream Builder City of Moncton Dream Builder CN Dream Builder Corey Graig Group Dream Builder In Loving Memory of Esther Fine - Isadore and Fami Dream Builder FMH Management Ltd. Dream Builder Bud and Betty Fogarty Dream Builder Harrison Trimble High School Students Assoc. Dream Builder Hillsborough Fashions Dream Builder David and Nancy Holt Dream Builder Kinsmen Club of Moncton Dream Builder Estate of Marion E. Kirk Dream Builder Len and Peggy Lockhart Dream Builder Lounsbury Company Ltd. Dream Builder Estate of Cecilia M. and Walter J. Lutes Dream Builder Ronald E. and Margurite (Peg) MacBeath Dream Builder Jon and Leslie Manship Dream Builder Freda and William Marr Dream Builder Estate of Elspeth Ross McBeath Dream Builder Modern Enterprises Dream Builder Moncton Lions Club Inc. -

The Petitcodiac River Watershed Preliminary Water Classification Report
The Petitcodiac River Watershed Preliminary Water Classification Report By: Eric R Arseneau Project Coordinator P.O. Box 23046 Moncton, N.B., E1A 6S8 Tel (506) 384-3369 Fax (506) 854-4824 [email protected] Your Environmental Trust Fund at Work ! Votre Fonds en fiducie pour l’Environnement au Travail ! FOREWORD The summation of this report is the result of 4 years of study relating to the water quality found within the Petitcodiac River watershed. Like the environment that we live in, this is a living document. Until this report is submitted to the Minister of Environment and Local Government, the citizens residing within this watershed still have a say in what goals they see attainable in the future for this area. This document should be viewed as a road map to what lies ahead in terms of protecting, rehabilitating and maintaining all of our water resources that touch every part of our lives. As you will see in this report, some watercourses are more impacted than others. With this in mind, it is important to remember that anything can be improved as long as we put the necessary effort into it. This report would not have been possible if not for the contribution of our dedicated volunteers who gathered water samples throughout the years, taking time during their weekends to help promote this project. For this, PWMG is forever grateful. A special thanks also goes out to all the field technicians that have worked for this organization since 1997 Georges Brun, Kevin Murphy, Pascal Levesque, Annie Haché and Shawn Craig as well as the previous coordinators Isabelle Frenette and Sandra Cashin. -
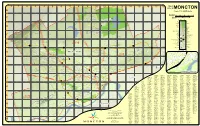
Moncton Map Carte.Pdf
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 City of Ville deMONCTON B Sc a le 1:16,000 Éc helle Kilo m etres Kilomètres 0 0.5 Jl 1 2 Miles 6 0 0.5 1 C 2 Milles MaC jo r Attra c tio n Tra il y p r Majeor Attraction Sentier s s T r e Pa rk e ) Buildin g Park Édifice Wa rd Bo un da ry M Open Wa ter a i Royal Oaks Estates Quartier n Eaux libres & Golf Course / Terrain de golf Go lf Co urse 0 s 8 Terrain de golf Mud Fla t Strea m D Mud Flat Ruisseau Irishtown Nature Park Parc naturel d'Irishtown e Cem etery Grav Ý Cimetière Sc ho o l Churc h n École å catio æ Edu Église Réservoir U n der Co n struc tio n Ro a d Route en construction Ü Syn a go gue ▐ Synagogue McLaughlin Ma rsh ge Marsh Oran City Ha ll Reservoir Magnetic Hill Concert Site ñ City Hall Place des Spectacle Magnetic Hill Ra ilwa y Chemin de fer Lakeside Golf VIA Ra il 8 Prin c ipa l Highwa y Ma rker & I3 2 15 E Country Club VIA Rail Autoroute principale Airpo rt Jl Pub lic Wo rks Aéroport k Travaux publics Parc thématique de Magnetic Hill Ra m p Theme Park Ramp Highwa y Tra n s-Ca n a da Highwa y Ma rker 446 Autoroute Autoroute transcanadienne 2 Aren a Lib ra ry 490 ²³ te Aréna Ska IH Bibliothèque d Crandall £ Rea Po lic e Sta tio n R University C p Lo c a l Ro a d Ward M nd loo e Poste de police P eg L D Locale Rue a 126 n Magnetic Hill Co llec to r Highwa y Ma rker y s Quartier Hennesse Golf & Country Club Artère collectrice ¨! Fire Depa rtm en t F 4 115 Poste d'incendie 2 Ho spita l IC 450 462 Hôpital M all Æa Bus Sta tio n 452 Sho ppin g Area Gare d'autobus 454 I< a 465 -

Jaguar Cubs Are Born at the Magnetic Hill
September 5, 2019 For immediate release Magnetic Hill Zoo shares first precious photos of 2 baby jaguars born at Magnetic Hill Zoo on August 22 These are the first cubs (both males) for mother BJ and father Rio, born on the morning of August 22, 2019. Animal care staff at the Zoo always hope that any mammal born at the zoo receives the care required from mom. Sometimes, however, that is not the case and keepers must step in to assist. During their first veterinary check, it was obvious that one of the cubs was considerably smaller than the other. After a couple of days, animal care staff was concerned by his smaller size and lower growth curve. Staff began supplementing the smaller cub with some extra milk during weigh in time and immediately returning to mom and his brother. This process allows us to ensure proper behaviour development with mom and brother while ensuring a healthy growth. Zoo director Jill Marvin, said, “The cubs’ eyes are open, they are gaining weight and they are becoming steadier on their paws. The cubs first 30 days are still critical but we felt it important to share this exciting news and progress with our Moncton zoo supporters. We are very excited with the births of these two new additions and look forward to them being out for guests to see. However, it will not be for another couple weeks before they begin to venture out of their den and several weeks before they will be ready to venture to their outside exhibit for the visitors.” Female jaguars can have litters of one to four cubs, which are blind and helpless at birth. -

COPYRIGHTED MATERIAL the Battery (St
INDEX See also Accommodations and Restaurant indexes, below. GENERAL Art Gallery of Nova Scotia Bed & breakfasts (B&Bs), 48 (Halifax), 108 Bell, Alexander Graham, 149 Art Gallery of Nova Scotia National Historic Site ARP, 38 A (Western Branch; (Baddeck), 150 Acadia, CSS (Halifax), 107 Yarmouth), 85 Bell Island, 319 The Acadian Coast, 82–84 Art Gallery of Nova Scotia Bell Island Community Acadian Lines, 33 Shop (Halifax), 112 Museum and Mine Tour, The Acadian Peninsula, Atlantic Film Festival 319 211–215 (Halifax), 105 Berry Hill, 283 Acadia University (Wolfville), Atlantic Jazz Festival Berry Hill Pond Trail, 282–283 69 (Halifax), 105 Biking and mountain biking, Accommodations, 6–7, 40, Atlantic Salmon Interpretive 41 43, 47–50. See also Centre (St. Andrews), 175 New Brunswick, 167, 180, Accommodations Indexes ATMs (automated-teller 194, 189–190, 202, 206, Adventure travel, 42 machines), 35–36 209, 216 Air travel, 31, 33 Auk Island Winery Newfoundland, 268–269, Aitkens Pewter (Fredericton), (Twillingate), 292 274 196 Automobile organizations, Nova Scotia, 65, 77, 79–80, Alexander Graham Bell 326 92, 145–146, 162 National Historic Site Avonlea (Cavendish), 227 Prince Edward Island, 222, (Baddeck), 150 238, 258 American Express, 326 The Bird House and traveler’s checks, 36 accalieu Island, 307–308 Binocular Shop (St. John’s), Amherst, 126–127 B Baccalieu Trail, 306–311 318 Amherst Marsh, 126 Backcountry camping, Nova Birds and bird-watching, Amos Pewter (Mahone Bay), Scotia (Kejimkujik National 19–22 98 Park), 78 New Brunswick, 167, 199, Anglican Cathedral of St. Backcountry hiking and 200 John the Baptist (St. backpacking, New Newfoundland, 269, 308 John’s), 314 Brunswick, 167 Nova Scotia, 65, 155–156 Anglo Rustico, 230 Backroads, 39, 42 Prince Edward Island, 253 Annapolis Royal, 72–76 Baddeck, 136, 149–152 Black bears, 16 Annapolis Royal Tidal Power Baker’s Brook Falls Trail, 283 Blockhouse National Historic Generation Station, 72 Balancing Rock, 80 Site (St. -

Moncton Moncton
W Timberline h i t Via Roma f i London e Longfellow l d T r i t e s 5 Front Mountain Gorge 6 7 Eaglewood 8 4 Seabury Site de concerts MAGNETIC HILL Oakcroft Mountain 1 Concert Site Magic Mountain Prayer Garden Kervin Congressional Jardin de la prière Irishtown Nature Park 2 3 Parc naturel d’Irishtown En Sedona sle y St. Andrews Royal Oaks Champ d'aviation King's B Mountain Woods ridge Golf Club McEwen d y r r r o 26 Golf Club f e Airfield g b Woods n w Te a e e CarlsonL Tim Chateau N e d r 450 o f Crandall Sam Rockley aw r University Snead Auberry C Réservoir Royal Oak s 08 Titus Sandy McLaughlin King Fisher Grosbeak Purple Charles Lutes Mountain Colby Richardson Amity Finch Réservoir Monique Reservoir Blue J Irishtown Casino Lauvrière a Seagull y Sparrow 1 Willowgrove Reservoir Pheasant N.B. Heron B P BlueAsh Chickadee Hummingbird L CypressCedarwood Tree 9 Sandpiper M a W a W Spruce Wood i I r n y n i 115490 Avery h a b c e Fir s A g Elmwood Reginald l i h 2 t t e d Overlea t n O 6 l l s e 5 r Sunshine e w e r a u Woodhaven e p r l w e o t y o d t B 3 e e 7 Glen Abbey o o H Cumberland e n i r B d 9 Crowbush o c d Southpine w 4 8 n a d h Oak Hill 452 Renshaw o r o C o S n Candlewood Firestone m Northwood Cherrylawn Cedargrove d A M a an Zurich c s ia LaGenevau Zurich D L Vercasca r n Jayden o n o o o e Muirfield N.B. -

The Shopping Experience Comes First at ANBL If, As the Adage Goes, the Customer Is Always Right, Then He Or She Is Also Increasingly Discerning and Informed
TRAVEL & TOURISM DIRECTORY The shopping experience comes first at ANBL If, as the adage goes, the customer is always right, then he or she is also increasingly discerning and informed. That’s something ANBL understands implicitly with every bottle or can it sells to customers across the province. These days, offering exciting shopping opportunities is key. Not only does ANBL offer product diversity and great value, it also strives to provide unique shopping experiences. Three of these truly unique experiences include the Craft Beer Room in Fredericton, the Depot in Salisbury, and the recently opened EXPÉRIENCE by ANBL in Moncton. EXPÉRIENCE by ANBL How do you DEPOT? Craft Beer Room Leah Anderson, Manager of the Situated a little outside of Moncton (TCH Head west on the Trans-Canada EXPÉRIENCE by ANBL boutique, says 2, Exit 433), is ANBL’s Depot store. This Highway, and you may find yourself at she, for one, is “beyond excited. This store also beckons consumers with its own the Craft Beer Room, located in a historic is New Brunswick’s first specialty store set of unique experiences. “The Depot is property in downtown Fredericton. It for wine, spirits, and beer. We wanted a unique concept,” says Store Manager boasts the largest craft beer selection in to create something unique, accessible Cheryl Black. “It is the only store of its the province—more than 200 brands. and fun for everyone—from beginner kind in Atlantic Canada. We like to say Bianca Brophy is the key buyer to connoisseur.” This new boutique is that Depot is the Costco of our industry. -

SUMMARY: Environmental Impact Assessment Report for Modifications to the Petitcodiac River Causeway
Summary of the Environmental Impact Assessment Report for Modifications to the Petitcodiac River Causeway October 2005 Prepared by the New Brunswick Department of Environment and Local Government EIA Summary - Modifications to the Petitcodiac River Causeway October 2005 Table of Contents 1.0 INTRODUCTION .................................................................................................................................................1 2.0 BACKGROUND....................................................................................................................................................1 3.0 ENVIRONMENTAL ASSESSMENT METHODS ............................................................................................2 4.0 PUBLIC, STAKEHOLDER, ABORIGINAL COMMUNITY MEETINGS AND REGULATORY CONSULTATION.......................................................................................................................................................2 5.0 DESCRIPTION OF PAST AND EXISTING ENVIRONMENT ......................................................................3 5.1 CAUSEWAY AND CONTROL STRUCTURE ..............................................................................................................3 5.2 PHYSICAL CHARACTERISTICS OF THE PETITCODIAC RIVER ESTUARY..................................................................3 General .................................................................................................................................................................3 Tidal Bore -

ASHRAE Staff Report 2016 Region II CRC Moncton, NB
ASHRAE Region 2 CRC 2016 Moncton, New Brunswick, Canada CRC 2016 MINUTES 58 th Annual Region 2 CRC REGION 2 CRC 2016 MINUTES MONCTON, NEW BRUNSWICK, CANADA at DELTA BEAUSEJOUR HOTEL 750 MAIN STREET MONCTON NEW BRUNSWICK, CANADA Friday August 26, 2016 to Sunday August 28, 2016 Host: NB/PEI Chapter #117 MINUTES APPROVED At CRC 2017 Montreal Fri Aug 25/2017 CRC 2016 Minutes - Page 1 of 18 2016-CRC_Minutes 1 ASHRAE Region 2 CRC 2016 Moncton, New Brunswick, Canada CRC 2016 MINUTES 58 th Annual Region 2 CRC Action Items from the CRC 2016 Action Description Owner Timeline Item 1 Regional newsletter to be prepared (approx 3 to 3 per year) to ARC Ongoing improve communication to chapters. Ibraham Semhat 2 Regional manual to be completed and posted to web site. Completed 3 A Regional speakers list to be prepared and added to Regional web RVC CTTC site. Daniel Robert 4 Regional Manual of Operations to have added RVC visit responsibilities (who arranges and pays for travel, hotel, meals) 5 new stationery letterhead for ASHRAE Research Canada [ARC] Daryl Boyce to incorporating the new ASHRAE logo and name of the check into Corporation. creation of letterhead CRC 2016 Minutes - Page 2 of 18 2016-CRC_Minutes 2 ASHRAE Region 2 CRC 2016 Moncton, New Brunswick, Canada CRC 2016 MINUTES 58 th Annual Region 2 CRC ORIENTATION SESSION – Friday Aug 26, 2016 8:35am The following attended the Orientation Session CHAPTER DELEGATE ALTERNATE #100 Halifax Chris Theriault Conan Baker #117 NB/PEI Sharlene Innes Robert Hoadley #013 La Ville de Quebec Xavier Dion Ouellet -

2013 Annual Report City of Moncton
2013 Annual Report City of Moncton Moncton, New Brunswick, Canada Year ending December 31, 2013 City of Moncton 2013 Annual Report for Fiscal Year Ending December 31, 2013 Prepared by: Corporate Communications Department Finance and Administration Department (506) 853-3566 Moncton City Hall 655 Main Street, Moncton, NB E1C 1E8 Canadian Award for Financial Reporting The Government Finance Officers Association of the United States and Canada (GFOA) awarded a Canadian Award for Financial Reporting to the City of Moncton for its annual financial report for the fiscal year ended December 31, 2012. The Canadian Award for Financial Reporting program was established to encourage municipal governments throughout Canada to publish high quality financial reports and to provide peer recognition and technical guidance for officials preparing these reports. In order to be awarded this Canadian Award, for Financial Reporting, a government unit must publish an easily readable and efficiently organized annual financial report, whose contents conform to program standards. Such reports should go beyond the minimum requirements of generally accepted accounting principles and demonstrate an effort to clearly enhance an understanding of financial reporting by municipal governments, and address user needs. A Canadian Award for Financial Reporting is valid for a period of one year only. We believe our current report continues to conform to the Canadian Award for Financial Reporting program requirements and as such we are submitting it to the GFOA for consideration -

Lou Macnarin School June Newsletter 2014
Lou MacNarin School June Newsletter 2014 Visit our WEB PAGE at http://web1.nbed.nb.ca/sites/ASD-E/schools/loumacnarin/Pages/default.aspx May 28th – Lewisville Middle School Scavenger Hunt for Grade 5 students May 30th – Our Volunteer Celebration -in the library on Friday at breakfast. The celebration recognizes the volunteers’ contributions to the school. June 5 – Grades 3 – 5 Field Trip – Centennial Park June 6 – Grades 3 – 5 Field Trip – Centennial Park June 6 Pre Kindergarten Orientation Day. No School for Kindergarten students. June 6 Library Books need to be returned as soon as possible. Our collection needs to be shelved once all repairs to books are completed. Please take a minute to look around your house for both library and classroom books. Library Books are stamped on the inside covers and have a school barcode on the front. Most classroom books are stamped as well or carry the teacher's name on the inside cover. We appreciate your help with this. June 6 Deadline for applications for the Grade 4 In Harmony Music Program June 9 – 13 – Last week for PALS volunteers. Thanks to everyone who was willing and able to help out this year! June 10 – District Track and Field Day. Students on the team will be going to the event by bus. Parents are required to pick up their children by 3:00pm at the University of Moncton Stadium. June 11 – Grade 2 Swimming Field Trip Kindergarten Activity – MY GYM June 12 – Grade 4 Field Trip Cape Jourimain Grade 5 Visit To Lewisville Middle School June 13 - Dieppe Library will visit the school to discuss summer reading programs.