Supplementary Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
PROBING THE INTERACTION OF ASPERGILLUS FUMIGATUS CONIDIA AND HUMAN AIRWAY EPITHELIAL CELLS BY TRANSCRIPTIONAL PROFILING IN BOTH SPECIES by POL GOMEZ B.Sc., The University of British Columbia, 2002 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Experimental Medicine) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) January 2010 © Pol Gomez, 2010 ABSTRACT The cells of the airway epithelium play critical roles in host defense to inhaled irritants, and in asthma pathogenesis. These cells are constantly exposed to environmental factors, including the conidia of the ubiquitous mould Aspergillus fumigatus, which are small enough to reach the alveoli. A. fumigatus is associated with a spectrum of diseases ranging from asthma and allergic bronchopulmonary aspergillosis to aspergilloma and invasive aspergillosis. Airway epithelial cells have been shown to internalize A. fumigatus conidia in vitro, but the implications of this process for pathogenesis remain unclear. We have developed a cell culture model for this interaction using the human bronchial epithelium cell line 16HBE and a transgenic A. fumigatus strain expressing green fluorescent protein (GFP). Immunofluorescent staining and nystatin protection assays indicated that cells internalized upwards of 50% of bound conidia. Using fluorescence-activated cell sorting (FACS), cells directly interacting with conidia and cells not associated with any conidia were sorted into separate samples, with an overall accuracy of 75%. Genome-wide transcriptional profiling using microarrays revealed significant responses of 16HBE cells and conidia to each other. Significant changes in gene expression were identified between cells and conidia incubated alone versus together, as well as between GFP positive and negative sorted cells. -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Comparison of Orthologs Across Multiple Species by Various Strategies
COMPARISON OF ORTHOLOGS ACROSS MULTIPLE SPECIES BY VARIOUS STRATEGIES BY HUI LIU DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biophysics and Computational Biology in the Graduate College of the University of Illinois at Urbana-Champaign, 2014 Urbana, Illinois Doctoral Committee: Professor Eric Jakobsson, Chair, Director of Research Professor Gene E. Robinson Associate Professor Saurabh Sinha Assistant Professor Jian Ma Abstract Thanks to the improvement of genome sequencing technology, abundant multi-species genomic data now became available and comparative genomics continues to be a fast prospering filed of biological research. Through the comparison of genomes of different organisms, we can understand what, at the molecular level, distinguishes different life forms from each other. It shed light on revealing the evolution of biology. And it also helps to refine the annotations and functions of individual genomes. For example, through comparisons across mammalian genomes, we can give an estimate of the conserved set of genes across mammals and correspondingly, find the species-specific sets of genes or functions. However, comparative genomics can be feasible only if a meaningful classification of genes exists. A natural way to do so is to delineate sets of orthologous genes. However, debates exist about the appropriate way to define orthologs. It is originally defined as genes in different species which derive from speciation events. But such definition is not sufficient to derive orthologous genes due to the complexity of evolutionary events such as gene duplication and gene loss. While it is possible to correctly figure out all the evolutionary events with the true phylogenetic tree, the true phylogenetic tree itself is impractical to be inferred. -

Identification of Transcriptional Mechanisms Downstream of Nf1 Gene Defeciency in Malignant Peripheral Nerve Sheath Tumors Daochun Sun Wayne State University
Wayne State University DigitalCommons@WayneState Wayne State University Dissertations 1-1-2012 Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors Daochun Sun Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Recommended Citation Sun, Daochun, "Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors" (2012). Wayne State University Dissertations. Paper 558. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. IDENTIFICATION OF TRANSCRIPTIONAL MECHANISMS DOWNSTREAM OF NF1 GENE DEFECIENCY IN MALIGNANT PERIPHERAL NERVE SHEATH TUMORS by DAOCHUN SUN DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2012 MAJOR: MOLECULAR BIOLOGY AND GENETICS Approved by: _______________________________________ Advisor Date _______________________________________ _______________________________________ _______________________________________ © COPYRIGHT BY DAOCHUN SUN 2012 All Rights Reserved DEDICATION This work is dedicated to my parents and my wife Ze Zheng for their continuous support and understanding during the years of my education. I could not achieve my goal without them. ii ACKNOWLEDGMENTS I would like to express tremendous appreciation to my mentor, Dr. Michael Tainsky. His guidance and encouragement throughout this project made this dissertation come true. I would also like to thank my committee members, Dr. Raymond Mattingly and Dr. John Reiners Jr. for their sustained attention to this project during the monthly NF1 group meetings and committee meetings, Dr. -

Identification of RNA Bound to the TDP-43 Ribonucleoprotein Complex in the Adult Mouse Brain
Identification of RNA bound to the TDP-43 ribonucleoprotein complex in the adult mouse brain Running Title: Identification of RNA bound to TDP-43 Ramesh K. Narayanan1, Marie Mangelsdorf1, Ajay Panwar1, Tim J. Butler1, Peter G. Noakes1,2, Robyn H. Wallace1,3* 1Queensland Brain Institute, 2School of Biomedical Sciences, 3School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, QLD, 4072, Australia *Corresponding author: [email protected] Objectives. Cytoplasmic inclusions containing TDP-43 are a pathological hallmark of several neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. TDP-43 is an RNA binding protein involved in gene regulation through control of RNA transcription, splicing and transport. However, the function of TDP-43 in the nervous system is largely unknown and its role in the pathogenesis of ALS is unclear. The aim of this study was to identify genes in the central nervous system that are regulated by TDP-43. Methods. RNA-immunoprecipitation with anti-TDP-43 antibody, followed by microarray analysis (RIP- chip), was used to isolate and identify RNA bound to TDP-43 protein from mouse brain. Results. This analysis produced a list of 1,839 potential TDP-43 gene targets, many of which overlap with previous studies and whose functions include RNA processing and synaptic function. Immunohistochemistry demonstrated that the TDP-43 protein could be found at the presynaptic membrane of axon terminals in the neuromuscular junction in mice. Conclusions. The finding that TDP-43 binds to RNA that codes for genes related to synaptic function, together with the localisation of TDP-43 protein at axon terminals, suggest a role for TDP-43 in the transport of synaptic mRNAs into distal processes. -

Qt38n028mr Nosplash A3e1d84
! ""! ACKNOWLEDGEMENTS I dedicate this thesis to my parents who inspired me to become a scientist through invigorating scientific discussions at the dinner table even when I was too young to understand what the hippocampus was. They also prepared me for the ups and downs of science and supported me through all of these experiences. I would like to thank my advisor Dr. Elizabeth Blackburn and my thesis committee members Dr. Eric Verdin, and Dr. Emmanuelle Passegue. Liz created a nurturing and supportive environment for me to explore my own ideas, while at the same time teaching me how to love science, test my questions, and of course provide endless ways to think about telomeres and telomerase. Eric and Emmanuelle both gave specific critical advice about the proper experiments for T cells and both volunteered their lab members for further critical advice. I always felt inspired with a sense of direction after thesis committee meetings. The Blackburn lab is full of smart and dedicated scientists whom I am thankful for their support. Specifically Dr. Shang Li and Dr. Brad Stohr for their stimulating scientific debates and “arguments.” Dr. Jue Lin, Dana Smith, Kyle Lapham, Dr. Tet Matsuguchi, and Kyle Jay for their friendships and discussions about what my data could possibly mean. Dr. Eva Samal for teaching me molecular biology techniques and putting up with my late night lab exercises. Beth Cimini for her expertise with microscopy, FACs, singing, and most of all for being a caring and supportive friend. Finally, I would like to thank Dr. Imke Listerman, my scientific partner for most of the breast cancer experiments. -

Solute Carrier Transporters (Slcs) As Possible Drug Targets for Cystic Fibrosis
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE QUÍMICA E BIOQUÍMICA Solute Carrier Transporters (SLCs) as Possible Drug Targets for Cystic Fibrosis Íris Lameiro Petinga Mestrado em Bioquímica Especialização em Bioquímica Médica Dissertação orientada por: Professora Doutora Margarida D. Amaral 2017 Acknowledgments/Agradecimentos Ao concluir este trabalho não posso deixar de agradecer a todas as pessoas que contribuíram de alguma maneira para a sua realização. Em primeiro lugar gostaria de agradecer à professora Margarida Amaral não só por me ter recebido no seu laboratório e grupo de investigação, mas também por toda a orientação e acompanhamento e acima de tudo confiança depositada. Não podia deixar também de agradecer ao professor Carlos Farinha por se ter mostrado sempre disponível para me ajudar, por toda a atenção, paciência e por me ter cativado para a área do estudo da Fibrose Quística desde muito cedo. Agradeço a todos os meus colegas de laboratório que sempre se mostraram disponíveis para me ajudar, todos eles à sua maneira. Primeiramente gostaria de agradecer à Verónica que tornou todos os infindáveis “Colony PCR” mais divertidos e me ajudou a seguir em frente quando tudo corria mal. À Sara cuja paciente infinitiva em me ensinar a trabalhar na cultura e grande parte das outras técnicas que utilizei neste trabalho, para além de responder sempre com boa disposição a todas as minhas dúvidas e questões que foram enumeras e muitas delas sem nexo, por vezes. À Madalena e aos seus maravilhosos cadernos de laboratório, sem dúvida a minha fonte de inspiração, mesmo longe esteve sempre disponível para responder às minhas dúvidas por mais chatas e aborrecidas que fossem e me fez crer desde o primeiro dia que seria possível. -
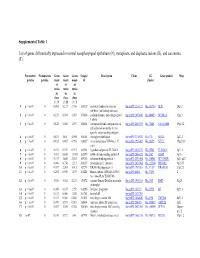
Supplemental Table 1 List of Genes Differentially Expressed In
Supplemental Table 1 List of genes differentially expressed in normal nasopharyngeal epithelium (N), metaplastic and displastic lesions (R), and carcinoma (T). Parametric Permutation Geom Geom Geom Unique Description Clone UG Gene symbol Map p-value p-value mean mean mean id cluster of of of ratios ratios ratios in in in class class class 1 : N 2 : R 3 : T 1 p < 1e-07 0 0.061 0.123 2.708 169329 secretory leukocyte protease IncytePD:2510171 Hs.251754 SLPI 20q12 inhibitor (antileukoproteinase) 2 p < 1e-07 0 0.125 0.394 1.863 163628 sodium channel, nonvoltage-gated IncytePD:1453049 Hs.446415 SCNN1A 12p13 1 alpha 3 p < 1e-07 0 0.122 0.046 1.497 160401 carcinoembryonic antigen-related IncytePD:2060355 Hs.73848 CEACAM6 19q13.2 cell adhesion molecule 6 (non- specific cross reacting antigen) 4 p < 1e-07 0 0.675 1.64 5.594 165101 monoglyceride lipase IncytePD:2174920 Hs.6721 MGLL 3q21.3 5 p < 1e-07 0 0.182 0.487 0.998 166827 nei endonuclease VIII-like 1 (E. IncytePD:1926409 Hs.28355 NEIL1 15q22.33 coli) 6 p < 1e-07 0 0.194 0.339 0.915 162931 hypothetical protein FLJ22418 IncytePD:2816379 Hs.36563 FLJ22418 1p11.1 7 p < 1e-07 0 1.313 0.645 13.593 162399 S100 calcium binding protein P IncytePD:2060823 Hs.2962 S100P 4p16 8 p < 1e-07 0 0.157 1.445 2.563 169315 selenium binding protein 1 IncytePD:2591494 Hs.334841 SELENBP1 1q21-q22 9 p < 1e-07 0 0.046 0.738 1.213 160115 prominin-like 1 (mouse) IncytePD:2070568 Hs.112360 PROML1 4p15.33 10 p < 1e-07 0 0.787 2.264 3.013 167294 HRAS-like suppressor 3 IncytePD:1969263 Hs.37189 HRASLS3 11q12.3 11 p < 1e-07 0 0.292 0.539 1.493 168221 Homo sapiens cDNA FLJ13510 IncytePD:64451 Hs.37896 2 fis, clone PLACE1005146. -

4211C3bea57068cc0a32976fb2
International Journal of Molecular Sciences Article Multiple Known Mechanisms and a Possible Role of an Enhanced Immune System in Bt-Resistance in a Field Population of the Bollworm, Helicoverpa zea: Differences in Gene Expression with RNAseq Roger D. Lawrie 1,2 , Robert D. Mitchell III 3, Jean Marcel Deguenon 2, Loganathan Ponnusamy 2 , Dominic Reisig 4, Alejandro Del Pozo-Valdivia 4, Ryan W. Kurtz 5 and R. Michael Roe 1,2,* 1 Department of Biology/Environmental and Molecular Toxicology Program, 850 Main Campus Dr, North Carolina State University, Raleigh, NC 27695, USA; [email protected] 2 Department of Entomology and Plant Pathology, Campus Box 7647, 3230 Ligon Street, North Carolina State University, Raleigh, NC 27695, USA; [email protected] (J.M.D.); [email protected] (L.P.) 3 Knipling-Bushland US Livestock Insects Research Laboratory Genomics Center, 2700 Fredericksburg Road, United States Department of Agriculture-Agricultural Research Service, Kerrville, TX 78028, USA; [email protected] 4 Department of Entomology and Plant Pathology, Vernon G. James Research & Extension Center, 207 Research Station Road, Plymouth, NC 27962, USA; [email protected] (D.R.); [email protected] (A.D.P.-V.) 5 Cotton Incorporated, 6399 Weston Parkway, Cary, NC 27513, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-515-4325 Received: 31 July 2020; Accepted: 1 September 2020; Published: 7 September 2020 Abstract: Several different agricultural insect pests have developed field resistance to Bt (Bacillus thuringiensis) proteins (ex. Cry1Ac, Cry1F, etc.) expressed in crops, including corn and cotton. In the bollworm, Helicoverpa zea, resistance levels are increasing; recent reports in 2019 show up to 1000-fold levels of resistance to Cry1Ac, a major insecticidal protein in Bt-crops. -

Human Social Genomics in the Multi-Ethnic Study of Atherosclerosis
Getting “Under the Skin”: Human Social Genomics in the Multi-Ethnic Study of Atherosclerosis by Kristen Monét Brown A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Epidemiological Science) in the University of Michigan 2017 Doctoral Committee: Professor Ana V. Diez-Roux, Co-Chair, Drexel University Professor Sharon R. Kardia, Co-Chair Professor Bhramar Mukherjee Assistant Professor Belinda Needham Assistant Professor Jennifer A. Smith © Kristen Monét Brown, 2017 [email protected] ORCID iD: 0000-0002-9955-0568 Dedication I dedicate this dissertation to my grandmother, Gertrude Delores Hampton. Nanny, no one wanted to see me become “Dr. Brown” more than you. I know that you are standing over the bannister of heaven smiling and beaming with pride. I love you more than my words could ever fully express. ii Acknowledgements First, I give honor to God, who is the head of my life. Truly, without Him, none of this would be possible. Countless times throughout this doctoral journey I have relied my favorite scripture, “And we know that all things work together for good, to them that love God, to them who are called according to His purpose (Romans 8:28).” Secondly, I acknowledge my parents, James and Marilyn Brown. From an early age, you two instilled in me the value of education and have been my biggest cheerleaders throughout my entire life. I thank you for your unconditional love, encouragement, sacrifices, and support. I would not be here today without you. I truly thank God that out of the all of the people in the world that He could have chosen to be my parents, that He chose the two of you. -

Analysis of the Impact of Pufas on Placental Gene Expression V2.28 Uploadx
TECHNISCHE UNIVERSITÄT MÜNCHEN Lehrstuhl für Ernährungsmedizin Analysis of the Impact of Polyunsaturated Fatty Acids (PUFAs) on Placental Gene Expression Eva-Maria Sedlmeier Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. M. Klingenspor Prüfer der Dissertation: 1. Univ.-Prof. Dr. J. J. Hauner 2. Univ.-Prof. Dr. H. Daniel Die Dissertation wurde am 17.10.2013 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 12.12.2013 angenommen. Table of contents Table of contents Table of contents ....................................................................................................... I Summary .................................................................................................................. VI Zusammenfassung ................................................................................................ VIII 1. Introduction ........................................................................................................ 1 1.1. Fetal programming - a strategy to prevent the obesity epidemic? ...........................................1 1.1.1. The obesity epidemic ........................................................................................................1 1.1.2. Fetal programming -

Autism Gene Ube3a and Seizures Impair Sociability by Repressing VTA Cbln1
Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Krishnan, V., D. C. Stoppel, Y. Nong, M. A. Johnson, M. J. Nadler, E. Ozkaynak, B. L. Teng, et al. 2017. “Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1.” Nature 543 (7646): 507-512. doi:10.1038/nature21678. http://dx.doi.org/10.1038/ nature21678. Published Version doi:10.1038/nature21678 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:34491817 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Nature. Manuscript Author Author manuscript; Manuscript Author available in PMC 2017 September 15. Published in final edited form as: Nature. 2017 March 23; 543(7646): 507–512. doi:10.1038/nature21678. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1 Vaishnav Krishnan*,1, David C. Stoppel*,1,2,7, Yi Nong*,1,2, Mark A. Johnson1,2, Monica J.S. Nadler1,2, Ekim Ozkaynak1,2, Brian L. Teng1,2, Ikue Nagakura1,2, Fahim Mohammad2, Michael A. Silva1,2, Sally Peterson1,2, Tristan J. Cruz1,2, Ekkehard M. Kasper3, Ramy Arnaout2,4,5, and Matthew P. Anderson‡,1,2,6,7 1Department of Neurology, Beth Israel Deaconess