Biomechanical Aspects of Bone Microstructure in Vertebrates: Potential Approach to Paleontological Investigations 799
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Introduction
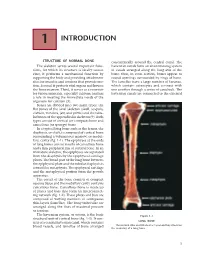
1 INTRODUCTION STRUCTURE OF NORMAL BONE concentrically around the central canal. The The skeleton serves several important func- haversian canals form an anastomosing system tions, for which its structure is ideally suited. of canals arranged along the long axis of the First, it performs a mechanical function by bone; thus, in cross section, bones appear as supporting the body and providing attachment round openings surrounded by rings of bone. sites for muscles and tendons that provide mo- The lamellae have a large number of lacunae, tion. Second, it protects vital organs and houses which contain osteocytes and connect with the bone marrow. Third, it serves as a reservoir one another through a series of canaliculi. The for various minerals, especially calcium, and has haversian canals are connected to the external a role in meeting the immediate needs of the organism for calcium (3). Bones are divided into two main types: the flat bones of the axial skeleton (skull, scapula, clavicle, vertebra, jaw, and pelvis) and the tubu- lar bones of the appendicular skeleton (9). Both types consist of cortical (or compact) bone and cancellous (or spongy) bone. In a typical long bone such as the femur, the diaphysis, or shaft, is composed of cortical bone surrounding a voluminous marrow, or medul- lary, cavity (fig. 1-1). The epiphyses at the ends of long bones consist mostly of cancellous bone and a thin peripheral rim of cortical bone. In an immature skeleton, the epiphyses are separated from the diaphysis by the epiphyseal cartilage plates. The broad part of the long bone between the epiphyseal plate and the tubular diaphysis is termed the metaphysis. -

Effect of the Combination of Ipriflavone and 1
PEDIATRIC DENTAL JOURNAL 14(1): 5–15, 2004 5 Effect of the combination of ipriflavone and 1␣-OH-D3 on debilitant bone in growing rats —Ultrastructural study of endochondral ossification— Kazushige Ueda, Ikuko Nishida, Bin Xia, Iwan Tofani, Jianguo Wang, Yasuhiro Nishikawa and Mitsutaka Kimura Department of Pediatric Dentistry, Kyushu Dental College 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu City, Fukuoka 803-8580, JAPAN Abstract Five-week-old male Wistar rats were used to study the effect of Key words dietary therapy with ipriflavone combined with 1␣-OH-D3. Ultrastructural alter- 1␣-OH-D3, ations in the metaphysis of debilitated tibia were observed in the growing rats. Growing rats, I. Light microscopy findings Ipriflavone, In the low-calcium diet • standard diet with supplementary ipriflavone and Metaphysis tibia 1␣-OH-D3 group, calcification of the chondral matrix and ossification were active, and the tibia grew normally as in the control group. II. Scanning electron microscopy findings In the low-calcium diet • standard diet with supplementary ipriflavone and 1␣-OH-D3 group, dense calcospherites, distinct chondral lacunae, regularly running collagen fibers, and distinct border lines were noted. III. Transmission electron microscopy findings In the low-calcium diet • standard diet with supplementary ipriflavone and 1␣-OH-D3 group we found that the osteoblasts were active, the ruffled border of osteoclast was decrease, indicated this osteoclast is inactive. In conclusion, insufficient calcium intake during the developmental period resulted in debilitated(3etaphysis tibia, whereas dietary therapy using combined ipriflavone and 1␣-OH-D3 promoted recovery. Introduction patients become refractory after several days of administration, which is known as the calcitonin Recently dietary therapies, physiologic active sub- escape phenomenon, therefore, it is not considered stances, exercise, and/or bone resorption inhibitors a suitable therapy for osteoporosis3). -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Bone Cartilage Dense Fibrous CT (Tendons & Nonelastic Ligaments) Dense Elastic CT (Elastic Ligaments)
Chapter 6 Content Review Questions 1-8 1. The skeletal system consists of what connective tissues? Bone Cartilage Dense fibrous CT (tendons & nonelastic ligaments) Dense elastic CT (elastic ligaments) List the functions of these tissues. Bone: supports the body, protects internal organs, provides levers on which muscles act, store minerals, and produce blood cells. Cartilage provides a model for bone formation and growth, provides a smooth cushion between adjacent bones, and provides firm, flexible support. Tendons attach muscles to bones and ligaments attach bone to bone. 2. Name the major types of fibers and molecules found in the extracellular matrix of the skeletal system. Collagen Proteoglycans Hydroxyapatite Water Minerals How do they contribute to the functions of tendons, ligaments, cartilage and bones? The collagen fibers of tendons and ligaments make these structures very tough, like ropes or cables. Collagen makes cartilage tough, whereas the water-filled proteoglycans make it smooth and resistant. As a result, cartilage is relatively rigid, but springs back to its original shape if it is bent or slightly compressed, and it is an excellent shock absorber. The extracellular matrix of bone contains collagen and minerals, including calcium and phosphate. Collagen is a tough, ropelike protein, which lends flexible strength to the bone. The mineral component gives the bone compression (weight-bearing) strength. Most of the mineral in the bone is in the form of hydroxyapatite. 3. Define the terms diaphysis, epiphysis, epiphyseal plate, medullary cavity, articular cartilage, periosteum, and endosteum. Diaphysis – the central shaft of a long bone. Epiphysis – the ends of a long bone. Epiphyseal plate – the site of growth in bone length, found between each epiphysis and diaphysis of a long bone and composed of cartilage. -

Dimensional Reconstruction of Haversian Systems in Human
Journal of Anatomy J. Anat. (2016) doi: 10.1111/joa.12430 Three-dimensional reconstruction of Haversian systems in human cortical bone using synchrotron radiation-based micro-CT: morphology and quantification of branching and transverse connections across age Isabel S. Maggiano,1,2 Corey M. Maggiano,2,3 John G. Clement,4 C. David L. Thomas,4 Yasmin Carter5 and David M. L. Cooper1 1Department of Anatomy and Cell Biology, University of Saskatchewan, Saskatoon, SK, Canada 2Department of Anthropology, University of West Georgia, Carrollton, GA, USA 3Department of Anthropology, University of Western Ontario, London, ON, Canada 4Melbourne Dental School, University of Melbourne, Melbourne, Vic., Australia 5Department of Radiology, University of Massachusetts Medical School, Worchester, MA, USA Abstract This study uses synchrotron radiation-based micro-computed tomography (CT) scans to reconstruct three- dimensional networks of Haversian systems in human cortical bone in order to observe and analyse interconnectivity of Haversian systems and the development of total Haversian networks across different ages. A better knowledge of how Haversian systems interact with each other is essential to improve understanding of remodeling mechanisms and bone maintenance; however, previous methodological approaches (e.g. serial sections) did not reveal enough detail to follow the specific morphology of Haversian branching, for example. Accordingly, the aim of the present study was to identify the morphological diversity of branching patterns and transverse connections, and to understand how they change with age. Two types of branching morphologies were identified: lateral branching, resulting in small osteon branches bifurcating off of larger Haversian canals; and dichotomous branching, the formation of two new osteonal branches from one. -

The Mechanoresponse of Bone Is Closely Related to the Osteocyte Lacunocanalicular Network Architecture
The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture Alexander Franciscus van Tola,b,1, Victoria Schemenza,b, Wolfgang Wagermaiera, Andreas Roschgera,c, Hajar Razia, Isabela Vitienesd,e, Peter Fratzla, Bettina M. Willied,e, and Richard Weinkamera,1 aDepartment of Biomaterials, Max Planck Institute of Colloids and Interfaces, 14476 Potsdam, Germany; bBerlin-Brandenburg School for Regenerative Therapies, Charité–Universitätsmedizin Berlin, 13353 Berlin, Germany; cDepartment of Chemistry and Physics of Materials, Paris Lodron University of Salzburg, A-5020 Salzburg, Austria; dResearch Centre, Shriners Hospital for Children-Canada, Montreal, H4A 0A9, Canada; and eDepartment of Pediatric Surgery, McGill University, Montreal, H3A 0G4, Canada Edited by Lia Addadi, Weizmann Institute of Science, Rehovot, Israel, and approved November 1, 2020 (received for review June 4, 2020) Organisms rely on mechanosensing mechanisms to adapt to changes materials, where the fluid-filled network should impart self-healing in their mechanical environment. Fluid-filled network structures not properties to the material (10, 11). only ensure efficient transport but canalsobeemployedformecha- In bone, fluid flow occurs in the lacunocanalicular network nosensation. The lacunocanalicular network (LCN) is a fluid-filled net- (LCN), a porous network of micrometer-sized lacunae con- work structure, which pervades our bones and accommodates a cell nected by roughly 300-nm-wide canals, called canaliculi (12, 13). network -

OSTEOCYTE SIGNALING and ITS EFFECTS on the ACTIVITES of OSTEOBLASTS and BREAST CANCER CELLS by Sina Ahandoust
OSTEOCYTE SIGNALING AND ITS EFFECTS ON THE ACTIVITES OF OSTEOBLASTS AND BREAST CANCER CELLS by Sina Ahandoust A Thesis Submitted to the Faculty of Purdue University In Partial Fulfillment of the Requirements for the degree of Master of Science in Biomedical Engineering Department of Biomedical Engineering at IUPUI Indianapolis, Indiana May 2021 THE PURDUE UNIVERSITY GRADUATE SCHOOL STATEMENT OF COMMITTEE APPROVAL Dr. Sungsoo Na, Chair Department of Biomedical Engineering Dr. Hiroki Yokota Department of Biomedical Engineering Dr. Jiliang Li Department of Biology Approved by: Dr. Joseph Wallace 2 ACKNOWLEDGMENTS I would like to appreciate my supervisor, Dr. Sungsoo Na, for his guidance and support. This study would not be possible without his careful direction, expertise, experience and encouragement. I would also like to thank the members of my thesis advisory committee, Dr. Hiroki Yokota and Dr. Jiliang Li for their support throughout my project. Finally, I would like to appreciate my family and friends for their support during my graduate studies. 3 TABLE OF CONTENTS LIST OF FIGURES ........................................................................................................................ 5 LIST OF ABBREVIATIONS ......................................................................................................... 7 ABSTRACT .................................................................................................................................... 8 1. INTRODUCTION ................................................................................................................. -

Korean Knee Society Terminology Book For
Korean Knee Society’s Terminology Book This edition first published 2015 © 2015 by Korean Knee Society. Published edition © 2015 Wiley Publishing Japan K.K. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN: 978-4-939028-29-8 Published by Wiley Publishing Japan K.K. Tokyo Office: Frontier Koishikawa Bldg. 4F, 1-28-1 Koishikawa, Bunkyo-ku, Tokyo 112-0002, Japan Telephone: 81-3-3830-1221 Fax: 81-3-5689-7276 Internet site: http://www.wiley.com/wiley-blackwell e-mail: [email protected] Printed and bound in Korea by JEIL PRINTECH 발 간 사 대부분의 의학 용어는 영어에서 유래되어 그 해석과 의미가 오 랜 시간 동안 계승 발전되어 왔습니다. 그러나 오랜 역사를 거 치면서 용어의 해석이 다양해져 교육 현장은 물론 임상에서도 여러 용어로 혼용되고 있어 ‘용어 통일’의 필요성이 지속적으로 대두되어 왔습니다. 이에 대한슬관절학회에서는 슬관절 분야 에서 많이 쓰이고 있는 용어들을 수집하여 용어를 재정비 하고 통일하는 사업을 추진하였습니다. 대한슬관절학회 교과서편찬위원회에서는 2013년 12월에 업무를 개시하여 1년 5 개월 동안 슬관절 분야의 대표적 영어 교과서의 색인, 대한정형외과학회 교과서 색 인 및 용어집, 대한슬관절학회 교과서 편찬시 논의 된 용어통일 리스트에서 용어를 수집하여 약 5,000 여개의 주요 용어를 정리하였습니다. 이번 용어 편찬 작업에서 주목할 만한 부분은 관련 전문가들은 물론 일반인들도 용 어 해석을 쉽게 찾아볼 수 있도록 어플리케이션을 제작하여 앱 스토어나 플레이 스 토어에서 무료로 다운로드 하여 볼 수 있도록 접근성을 높였다는 것입니다. 이번 용 어집 편찬 작업이 선생님들의 진료와 연구에 도움이 되고, 일반인들도 보다 쉽고 정 확하게 의료진들과 소통할 수 있길 바라며 앞으로 꾸준히 발전해 나갈 수 있도록 많 은 관심 바랍니다. -

Imaging the Bone Cell Network with Nanoscale Synchrotron Computed Tomography Alexandra Joita Pacureanu
Imaging the bone cell network with nanoscale synchrotron computed tomography Alexandra Joita Pacureanu To cite this version: Alexandra Joita Pacureanu. Imaging the bone cell network with nanoscale synchrotron computed tomography. Other. INSA de Lyon, 2012. English. NNT : 2012ISAL0001. tel-00778408 HAL Id: tel-00778408 https://tel.archives-ouvertes.fr/tel-00778408 Submitted on 20 Jan 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° d’ordre 2012ISAL0001 Année 2012 Thèse Imaging the bone cell network with nanoscale synchrotron computed tomography Présentée devant L’Institut National des Sciences Appliquées de Lyon Pour obtenir Le grade de docteur ÉCOLE DOCTORALE ELECTRONIQUE, ELECTROTECHNIQUE, AUTOMATIQUE Par Alexandra PACUREANU Soutenue le 19 janvier 2012 devant la commission d’examen Jury P. Laugier Directeur de recherche CNRS UPMC Paris M. Garreau Professeur Université de Rennes 1 J. Klein-Nulend Professeur Université d’Amsterdam J.Y. Buffière Professeur INSA de Lyon M. Ciuc Maitre de Conférences Université Politehnica Bucarest C. Muller Maitre de Conférences INSA de Lyon V. Buzuloiu Professeur Université Politehnica Bucarest F. Peyrin Directeur de recherche INSERM INSA de Lyon Laboratoire de recherche CREATIS Cette thèse est accessible à l'adresse : http://theses.insa-lyon.fr/publication/2012ISAL0001/these.pdf © [A. -

BONE TISSUE Osteoblasts
Department of Histology and Embryology, P. J. Šafárik University, Medical Faculty, Košice BONE TISSUE: Sylabus for foreign students Author: MVDr. Zuzana Jonecová, CSc. Revised by: prof. MUDr. Eva Mechírová, CSc. BONE TISSUE - structural component of bones - specialized connective tissue for support and protection - composition: - cells - mineralized intercellular matrix – bone matrix Histological composition of the bone tissue: 1. Bone matrix – intercellular substance a. Inorganic components → Ca, P, Mg, K, Na → hyproxyapatit crystals (65%) crystals lie alongside collagen fibrills and are surrounded by amorphous ground substance b. Organic components: Fibers : collagen fibers (collagen type I ) ! Amorphous ground substance : noncollagenous proteins GAG, PG, structural GP small regulatory proteins 2. Cells a. Osteoprogenitor cells (preosteoblasts) b. Osteoblasts (bone forming cells) c. Osteocytes (inactive osteoblast) d. Osteoclasts (bone resorbing cells) Osteoblasts – line endosteal and periosteal surfaces ( 1 layer) - cuboidal cells with basophilic cytoplasm (proteosynthesis) - deposit of unmineralized organic matrix –osteoid – produced at the surface of the bone tissue when new bone tissue is required FUNCTION : 1) synthesis of organic components of bone matrix (growth, response to fracture, remodelling) 2) regulate mineralization of bone matrix 3) regulate activity of bone resorption (osteoclasts) Produce : - collagen type I (95 %) - non-collagenous bone matrix proteins : GAG, proteoglycans (hyaluronan, chondroitin and keratan sulfate) -

Functions of the Haversian System
Functions of the Haversian System DONALD H. ENLOW Department of Anatomy, The University of Michigan, Ann Arbor, Michigan The Haversian system or osteone has associated with areas of re-location in mus- been traditionally adopted as a universal cle attachment on a growing bone, and in unit of structure in compact bone. The remodeling processes involving resorption basic functions and the structural signifi- of periosteal bone surfaces during nieta- cance of primary and secondary Haversian physeal reduction in diameter axd re- tissue, however, are poorly understood. gional changes in shape. The hypothesis Two explanations on the functional mean- is advanced that this type of Haversian ing of the secondary Haversian system system functions as an anchoring mecha- have been proposed. These are (a) the nism which can maintain muscle con- interpretation of the osteone as an ex- tinuity and attachment with bone during clusive response to stress, and (b) the such remodeling changes. All secondary interpretation of the secondary osteone as osteones, regardless of particular function, an exclusive structural result of mineral are structurally comparable and represent mobilization and redistribution. However, a product of internal reconstruction with- the characteristic absence of the Haver- in compact bone. sian system in the compact bone of many History. Leeuwenhoek (1678) was the vertebrate species, including widely used first to notice the microscopic canal sgs- experimental forms such as the white rat, tern in bone, and he reported his observa- and the characteristic patterns of distribu- tions to members of the Royal Society in tion of Haversian systems in the bone of a series of personal communications which those species which do possess these were later published. -

Shaking Stimuli Can Retard Accelerated Decline of Bone Strength of a Mouse Model Assumed to Represent a Postmenopausal Woman
Journal of Analytical Bio-Science Vol. 33, No 4 (2010) 〈Original article〉 Shaking stimuli can retard accelerated decline of bone strength of a mouse model assumed to represent a postmenopausal woman Kouji Yamada*, Kazuhiro Nishii and Takehiko Hida Summary In contrast in elderly people, if bone formation and resorption are out of balance, bone mineral density (BMD) diminishes leading to progressive development of osteoporosis, a cause of easy bone fracture. Standing still for prolonged periods leads to fatigue. Humans tend to avoid this by shifting the body weight unconsciously or consciously left and right or back and forth. Standing in an erect posture on a board that continuously shakes horizontally causes all the skeletal muscles of the body as well as the entire skeleton to act to restore balance. As a result of the application of such shaking–induced stimuli to a reduced–BMD mouse model of postmenopausal osteoporosis, rapid decline in the bone strength of the femur was suppressed. A shaking stimulus such as was used in this study could easily be applied to human beings in future once further study is conducted to validate the results. Key words: Osteogenesis, Bone resorption, Shaking, Physical therapy 1. Introduction (BMD) continues to increase for a while after cessation of linear growth. Generally, bone mass in A rise in average longevity along with improved humans reaches a peak in the late twenties to thirties, treatment for organ disorders and various diseases remaining in a static state for some time thereafter. has rendered age-associated bone mass reduction a With further aging, bone volume is maintained but great clinical issue.