Total Synthesis of (+)-Strychnine Via a [4+2]-Cycloaddition/Rearrangement Cascade
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract Multicomponent Reactions of Salicylaldehyde, Cyclic Ketones, and Arylamines Through Cooperative Enamine-Metal Lewis
ABSTRACT MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS by Ryan Gregory Sarkisian Multicomponent reactions (MCRs) are the most atom economic, highly selective, and convergent type of reaction. This allows for a reaction to have a wide scope and allows for maximization of the complexity of a product. Catalyzing these MCRs with asymmetric catalysis is a novel way to introduce stereocontrol into highly complex molecules with various functional groups. Asymmetric catalysis is considered the most efficient method for constructing highly functionalized optically active stereopure compounds. There are three pillars of asymmetric catalysis: biocatalysis, transition metal catalysis, and organocatalysis. This research focuses on two of these pillars, transition metal catalysis and organocatalysis, working cooperatively to catalyze this MCR. The focus is to educate or refresh the audience on the basic topics that make up the complexity of the MCRs being catalyzed by cooperative asymmetric catalysis. Ultimately to explore the cooperative catalysts used to synthesize both the racemic and asymmetric three fused ring products (9-((4-methoxyphenyl)amino)-2,3,4,4a,9,9a-hexahydro-1H-xanthen-4a-ol). MULTICOMPONENT REACTIONS OF SALICYLALDEHYDE, CYCLIC KETONES, AND ARYLAMINES THROUGH COOPERATIVE ENAMINE-METAL LEWIS ACID CATALYSIS A THESIS Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of -

Catalytic Direct Asymmetric Michael Reactions
ORGANIC LETTERS 2001 Catalytic Direct Asymmetric Michael Vol. 3, No. 23 Reactions: Taming Naked Aldehyde 3737-3740 Donors Juan M. Betancort and Carlos F. Barbas III* The Skaggs Institute for Chemical Biology and the Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037 [email protected] Received September 5, 2001 ABSTRACT Direct catalytic enantio- and diastereoselective Michael addition reactions of unmodified aldehydes to nitro olefins using (S)-2-(morpholinomethyl)- pyrrolidine as a catalyst are described. The reactions proceed in good yield (up to 96%) in a highly syn-selective manner (up to 98:2) with enantioselectivities approaching 80%. The resulting γ-formyl nitro compounds are readily converted to chiral, nonracemic 3,4-disubstituted pyrrolidines. The Michael reaction is generally regarded as one of the Typically, carbon nucleophiles that contain an active most efficient carbon-carbon bond forming reactions, and methylene center such as malonic acid esters, â-keto esters, studies concerning this reaction have played an important nitroalkanes, etc. have been studied in the Michael reaction. role in the development of modern synthetic organic Carbonyl compounds, and ketones in particular, have gener- chemistry.1 As the demand for optically active compounds ally only been used as donors following their preactivation has soared in recent years, much progress has been made in by conversion into a more reactive species such as enol or the development of asymmetric variants of this reaction, enamine equivalents.5,6 In these cases, additional synthetic providing for the preparation of Michael adducts with high enantiomeric purity.2 Though remarkable advances have been (3) (a) Chataigner, I.; Gennari, C.; Ongeri, S.; Piarulli, U.; Ceccarelli, S. -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -
![Synthesis of Densely Substituted Pyridine Derivatives from Nitriles by a Non-Classical [4+2] Cycloaddition/1,5-Hydrogen Shift Strategy](https://docslib.b-cdn.net/cover/3428/synthesis-of-densely-substituted-pyridine-derivatives-from-nitriles-by-a-non-classical-4-2-cycloaddition-1-5-hydrogen-shift-strategy-483428.webp)
Synthesis of Densely Substituted Pyridine Derivatives from Nitriles by a Non-Classical [4+2] Cycloaddition/1,5-Hydrogen Shift Strategy
Synthesis of densely substituted pyridine derivatives from nitriles by a non-classical [4+2] cycloaddition/1,5-hydrogen shift strategy Wanqing Wu ( [email protected] ) South China University of Technology https://orcid.org/0000-0001-5151-7788 Dandan He South China University of Technology Kanghui Duan South China University of Technology Yang Zhou South China University of Technology Meng Li South China University of Technology Huanfeng Jiang South China University of Technology Article Keywords: pyridine derivatives, organic synthesis, medicinal chemistry. Posted Date: March 3rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-258126/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract A novel strategy has been established to assemble an array of densely substituted pyridine derivatives from nitriles and o-substituted aryl alkynes or 1-methyl-1,3-enynes via a non-classical [4 + 2] cycloaddition along with 1,5-hydrogen shift process. The well-balanced anities of two different alkali metal salts enable the C(sp3)-H bond activation as well as the excellent chemo- and regioselectivities. This protocol offers a new guide to construct pyridine frameworks from nitriles with sp3-carbon pronucleophiles, and shows potential applications in organic synthesis and medicinal chemistry. Introduction Compounds containing pyridine core structures, not only widely exist in natural products, pharmaceuticals, and functional materials,1–7 but also serve as useful and valuable building blocks for metal ligands.8,9 For instance, pyridine derivative Actos I10 is a famous drug for the treatment of type 2 diabetes; Bi-(or tri-)pyridines II11–15 are often used as ligands in metal-catalyzed reactions; Kv1.5 antagonist III16 and alkaloid papaverine IV17 are two representative isoquinolines as a promising atrial- selective agent and a smooth muscle relaxant, respectively (Fig. -

TR-470: Pyridine (CASRN 110-86-1) in F344/N Rats, Wistar Rats, And
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF PYRIDINE (CAS NO. 110-86-1) IN F344/N RATS, WISTAR RATS, AND B6C3F1 MICE (DRINKING WATER STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 March 2000 NTP TR 470 NIH Publication No. 00-3960 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Technical Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -

Toxicological Profile for Pyridine
TOXICOLOGICAL PROFILE FOR PYRIDINE Agency for Toxic Substances and Disease Registry U.S. Public Health Service September 1992 ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. 1 1. PUBLIC HEALTH STATEMENT This Statement was prepared to give you information about pyridine and to emphasize the human health effects that may result from exposure to it. The Environmental Protection Agency (EPA) has identified 1,177 sites on its National Priorities List (NPL). Pyridine has been found at 4 of these sites. However, we do not know how many of the 1,177 NPL sites have been evaluated for pyridine. As EPA evaluates more sites, the number of sites at which pyridine is found may change. This information is important for you to know because pyridine may cause harmful health effects and because these sites are potential or actual sources of human exposure to pyridine. When a chemical is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment as a chemical emission. This emission, which is also called a release, does not always lead to exposure. You can be exposed to a chemical only when you come into contact with the chemical. You may be exposed to it in the environment by breathing, eating, or drinking substances containing the chemical or from skin contact with it. If you are exposed to a hazardous chemical such as pyridine, several factors will determine whether harmful health effects will occur and what the type and severity of those health effects will be. -

Comparative Total Syntheses of Strychnine
Comparative Total Syntheses of Strychnine N C D MacMillan Group Meeting N H O H A H B E Nathan Jui H N N H H F G July 22, 2009 O O O References: Pre-Volhardt: Bonjoch Chem. Rev. 2000, 3455. Woodward Tetrahedron, 1963, 247. Volhardt J. Am. Chem. Soc. 2001, 9324. Magnus J. Am. Chem. Soc. 1993, 8116. Martin J. Am. Chem. Soc. 2001, 8003. Overman J. Am. Chem. Soc. 1995, 5776. Bodwell Angew. Chem. Int. Ed. 2002, 3261. Kuehne J. Org. Chem. 1993, 7490. Mori J. Am. Chem. Soc. 2003, 9801. Kuehne J. Org. Chem. 1998, 9427. Shibasaki Tetrahedron 2004, 9569. Rawal J. Org. Chem. 1994, 2685. Fukuyama J. Am. Chem. Soc. 2004, 10246. Bosch, Bonjoch Chem. Eur. J. 2000, 655. Padwa Org. Lett. 2007, 279. History and Structure of (!)-Strychnine ! Isolated in pure form in 1818 (Pelletier and Caventou) ! Structural Determination in 1947 (Robinson and Leuchs) ! 24 skeletal atoms (C21H22N2O2) ! Isolated in pure form in 1818 (Pelletier and Caventou) N ! Over !25 07 -pruinbglisc,a 6ti-osntesr epoecrteaninteinrgs to structure ! Structural Determination in 1947 (Robinson and Leuchs) ! Notor!io u Ss ptoirxoicne (nletethr a(lC d-o7s) e ~10-50 mg / adult) N H H ! Over 250 publications pertaining to structure O O ! Comm!o nClyD uEs eridn gr osdyesntet mpoison ! Notorious poison (lethal dose ~10-50 mg / adult) ! Hydroxyethylidine Strychnos nux vomica ! $20.20 / 10 g (Aldrich), ~1.5 wt% (seeds), ~1% (blossoms) "For it's molecular size it is the most complex substance known." -Robert Robinson History and Structure of (!)-Strychnine ! 24 skeletal atoms (C21H22N2O2) N ! 7-rings, 6-stereocenters ! Spirocenter (C-7) N H H O O ! CDE ring system ! Hydroxyethylidine "For it's molecular size it is the most complex substance known." -Robert Robinson "If we can't make strychnine, we'll take strychnine" -R. -
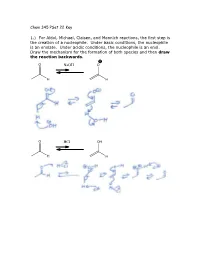
Chem 345 Pset 22 Key 1.) for Aldol, Michael, Claisen, and Mannich
Chem 345 PSet 22 Key 1.) For Aldol, Michael, Claisen, and Mannich reactions, the first step is the creation of a nucleophile. Under basic conditions, the nucleophile is an enolate. Under acidic conditions, the nucleophile is an enol. Draw the mechanism for the formation of both species and then draw the reaction backwards. O NaOH O H H O HCl OH H H 2.) In the case of a Mannich reaction, the electrophile (an imine) must also be made. Draw the mechanism for the forward and backward reaction: O MeNH2 N H H cat. AcOH H H 3.) When a secondary amine is used and the carbonyl has an enolizable proton, an enamine can be formed. Are enamines nucleophilic or electrophilic? Nucleophilic H O N N H2O H cat. AcOH H enamine N N H H enamine 4.) The following reaction is the Lobry-de-Bruyn-von-Ekenstein reaction. Don’t worry about the name. I only recently found out that that was the official name for the rearrangement leading from glucose to fructose or mannose and back again. I was taught it under a different name: the Carbohydrate Game. Under acidic or basic conditions, it is possible that the following sugars (glucose, fructose, and mannose) are in equilibrium with each other. Draw the acid catalyzed version and the base catalyzed version. (Hint: this is just a repeat of question 1.) O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose O O H H H2C OH + + HO H H3O H OH H3O O or or HO- HO- HO H HO H HO H H OH H OH H OH H OH H OH H OH H2C OH H2C OH H2C OH D-glucose D-fructose D-mannose The sugars are drawn in a Fisher projection (or what I think of is a dead cat projection). -

Thiocyanate Pyridine Complexes
W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2017 Structural Comparison of Copper(II) Thiocyanate Pyridine Complexes Joseph V. Handy College of WIlliam & Mary Follow this and additional works at: https://scholarworks.wm.edu/honorstheses Part of the Inorganic Chemistry Commons Recommended Citation Handy, Joseph V., "Structural Comparison of Copper(II) Thiocyanate Pyridine Complexes" (2017). Undergraduate Honors Theses. Paper 1100. https://scholarworks.wm.edu/honorstheses/1100 This Honors Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Structural Comparison of Copper(II) Thiocyanate Pyridine Complexes A thesis submitted in partial fulfillment of the requirement for the degree of Bachelor of Science in Chemistry from The College of William & Mary by Joseph Viau Handy Accepted for ____________________________ ________________________________ Professor Robert D. Pike ________________________________ Professor Deborah C. Bebout ________________________________ Professor David F. Grandis ________________________________ Professor William R. McNamara Williamsburg, VA May 3, 2017 1 Table of Contents Table of Contents…………………………………………………...……………………………2 List of Figures, Tables, and Charts………………………………………...…………………...4 Acknowledgements….…………………...………………………………………………………6 -

Efficient Synthesis of Novel Pyridine-Based Derivatives Via
molecules Article Efficient Synthesis of Novel Pyridine-Based Derivatives via Suzuki Cross-Coupling Reaction of Commercially Available 5-Bromo-2-methylpyridin-3-amine: Quantum Mechanical Investigations and Biological Activities Gulraiz Ahmad 1, Nasir Rasool 1,*, Hafiz Mansoor Ikram 1, Samreen Gul Khan 1, Tariq Mahmood 2, Khurshid Ayub 2, Muhammad Zubair 1, Eman Al-Zahrani 3, Usman Ali Rana 4, Muhammad Nadeem Akhtar 5 and Noorjahan Banu Alitheen 6,* 1 Department of Chemistry, Government College University Faisalabad, Faisalabad 38000, Pakistan; [email protected] (G.A.); [email protected] (H.M.I.); [email protected] (S.G.K.); [email protected] (M.Z.) 2 Department of Chemistry, COMSATS Institute of Information Technology, University Road, Tobe Camp, 22060 Abbottabad, Pakistan; [email protected] (T.M.); [email protected] (K.A.) 3 Chemistry Department, Faculty of Science, Taif University, 888-Taif, Saudi Arabia; [email protected] 4 Sustainable Energy Technologies Center, College of Engineering, P.O. Box 800, King Saud University, Riyadh 11421, Saudi Arabia; [email protected] 5 Faculty of Industrial Sciences & Technology, University Malaysia Pahang, Lebuhraya Tun, Razak 26300, Kuantan Pahang, Malaysia; [email protected] 6 Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, Serdang, Selangor Darul Ehsan 43400, Malaysia * Correspondence: [email protected] (N.R.); [email protected] (N.B.A.); Tel.: +92-332-7491790 (N.R.); +60-3-8946-7471 (N.B.A.); Fax: +92-41-9201032 (N.R.); +60-3-8946-7510 (N.B.A.) Academic Editor: Diego Muñoz-Torrero Received: 20 November 2016; Accepted: 6 January 2017; Published: 27 January 2017 Abstract: The present study describes palladium-catalyzed one pot Suzuki cross-coupling reaction to synthesize a series of novel pyridine derivatives 2a–2i, 4a–4i. -

Heterocyclic Chemistrychemistry
HeterocyclicHeterocyclic ChemistryChemistry Professor J. Stephen Clark Room C4-04 Email: [email protected] 2011 –2012 1 http://www.chem.gla.ac.uk/staff/stephenc/UndergraduateTeaching.html Recommended Reading • Heterocyclic Chemistry – J. A. Joule, K. Mills and G. F. Smith • Heterocyclic Chemistry (Oxford Primer Series) – T. Gilchrist • Aromatic Heterocyclic Chemistry – D. T. Davies 2 Course Summary Introduction • Definition of terms and classification of heterocycles • Functional group chemistry: imines, enamines, acetals, enols, and sulfur-containing groups Intermediates used for the construction of aromatic heterocycles • Synthesis of aromatic heterocycles • Carbon–heteroatom bond formation and choice of oxidation state • Examples of commonly used strategies for heterocycle synthesis Pyridines • General properties, electronic structure • Synthesis of pyridines • Electrophilic substitution of pyridines • Nucleophilic substitution of pyridines • Metallation of pyridines Pyridine derivatives • Structure and reactivity of oxy-pyridines, alkyl pyridines, pyridinium salts, and pyridine N-oxides Quinolines and isoquinolines • General properties and reactivity compared to pyridine • Electrophilic and nucleophilic substitution quinolines and isoquinolines 3 • General methods used for the synthesis of quinolines and isoquinolines Course Summary (cont) Five-membered aromatic heterocycles • General properties, structure and reactivity of pyrroles, furans and thiophenes • Methods and strategies for the synthesis of five-membered heteroaromatics