Radionuclides for the Great Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Strontium-90
EPA Facts about Strontium-90 What is strontium-90? The most common isotope of strontium is strontium-90. Radioactive strontium-90 is produced when uranium and plutonium undergo fission. Fission The time required for a radioactive substance to is the process in which the nucleus of a lose 50 percent of its radioactivity by decay is radionuclide breaks into smaller parts. Large known as the half-life. Strontium-90 has a half- amounts of radioactive strontium-90 were life of 29 years and emits beta particles of produced during atmospheric nuclear weapons relatively low energy as it decays. Yttrium-90, its tests conducted in the 1950s and 1960s. As a decay product, has a shorter half-life (64 hours) result of atmospheric testing and radioactive than strontium-90, but it emits beta particles of fallout, this strontium was dispersed and higher energy. deposited on the earth. How are people exposed to strontium- 90? What are the uses of strontium-90? Although external exposure to strontium-90 Strontium-90 is used in medical and agricultural from nuclear testing is of minor concern because studies. It is also used in thermoelectric devices environmental concentrations are low, that are built into small power supplies for use strontium in the environment can become part of the food chain. This pathway of exposure in remote locations, such as navigational beacons, remote weather stations, and space became a concern in the 1950s with the advent vehicles. Additionally, strontium-90 is used in of atmospheric testing of nuclear explosives. electron tubes, radioluminescent markers, as a With the suspension of atmospheric testing of radiation source in industrial thickness gauges, nuclear weapons, dietary intake has steadily and for treatment of eye diseases. -
Thallium-201 for Medical Use. I
THALLIUM-201 FOR MEDICAL USE. I E. Lebowitz, M. W. Greene, R. Fairchild, P. R. Bradley-Moore, H. 1. Atkins,A. N. Ansari, P. Richards,and E. Belgrave Brookhaven National Laboratory Thallium-201 merits evaluation for myocar tumors (7—9), the use of radiothallium should also dial visualization, kidney studies, and tumor be evaluated for this application. diagnosis because of its physical and biologic Thallium-201 decays by electron capture with a properties. A method is described for prepara 73-hr half-life. It emits mercury K-x-rays of 69—83 tion of this radiopharmaceutical for human use. keY in 98% abundance plus gamma rays of I 35 and A critical evaluation of 501T1 and other radio 167 keV in 10% total abundance. Because of its pharmaceuticals for myocardial visualization is good shelf-life, photon energies, and mode of decay, given. 201T1was the radioisotope of thallium chosen for development. Thallium-20 1 is a potentially useful radioisotope MATERIALS AND METHODS for various medical applications including myocardial Thallium-201 is produced by irradiating a natural visualization and possible assessment of physiology, thallium target in the external beam of the 60-in. as a renal medullary imaging agent, and for tumor Brookhaven cyclotron with 3 1-MeV protons. The detection. nuclear reaction is 203Tl(p,3n)201Pb. Lead-201 has The use of radiothallium in nuclear medicine was a half-life of 9.4 hr and is the parent of 201T1.The first suggested by Kawana, et al (1 ) . In terms of thallium target, fabricated from an ingot of 99.999% organ distribution (2) and neurophysiologic function pure natural thallium metal (29.5% isotopic abun (3), thallium is biologically similar to potassium. -
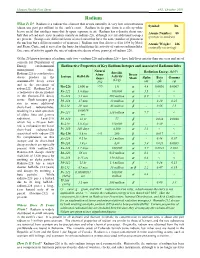
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

FT/P3-20 Physics and Engineering Basis of Multi-Functional Compact Tokamak Reactor Concept R.M.O
FT/P3-20 Physics and Engineering Basis of Multi-functional Compact Tokamak Reactor Concept R.M.O. Galvão1, G.O. Ludwig2, E. Del Bosco2, M.C.R. Andrade2, Jiangang Li3, Yuanxi Wan3 Yican Wu3, B. McNamara4, P. Edmonds, M. Gryaznevich5, R. Khairutdinov6, V. Lukash6, A. Danilov7, A. Dnestrovskij7 1CBPF/IFUSP, Rio de Janeiro, Brazil, 2Associated Plasma Laboratory, National Space Research Institute, São José dos Campos, SP, Brazil, 3Institute of Plasma Physics, CAS, Hefei, 230031, P.R. China, 4Leabrook Computing, Bournemouth, UK, 5EURATOM/UKAEA Fusion Association, Culham Science Centre, Abingdon, UK, 6TRINITI, Troitsk, RF, 7RRC “Kurchatov Institute”, Moscow, RF [email protected] Abstract An important milestone on the Fast Track path to Fusion Power is to demonstrate reliable commercial application of Fusion as soon as possible. Many applications of fusion, other than electricity production, have already been studied in some depth for ITER class facilities. We show that these applications might be usefully realized on a small scale, in a Multi-Functional Compact Tokamak Reactor based on a Spherical Tokamak with similar size, but higher fields and currents than the present experiments NSTX and MAST, where performance has already exceeded expectations. The small power outputs, 20-40MW, permit existing materials and technologies to be used. The analysis of the performance of the compact reactor is based on the solution of the global power balance using empirical scaling laws considering requirements for the minimum necessary fusion power (which is determined by the optimized efficiency of the blanket design), positive power gain and constraints on the wall load. In addition, ASTRA and DINA simulations have been performed for the range of the design parameters. -

Dr. A.A. El-Mohty Dr. Shoukar T.M. Atwa
Benha University Faculty of Science Chemistry Department RADIOCHEMICAL STUDIES ON THE SEPARATION OF IODINE- 131 AND RADIOIODINATION OF SOME ORGANIC COMPOUNDS A Thesis Submitted by MAHMOUD ABBAS ISMAIL MOHAMED Isotopes and Radioactive Generators Dep., Hot Labs. Center Atomic Energy Authority To Faculty of Science – Benha University Presented as partial fulfillment of The Degree of M.Sc In chemistry Supervised by Prof .Dr .H .A. Dessouki Prof . Dr .S .A .El -Bayoumy Prof. of Inorganic and Prof. of Radiochemistry Analytical Chemistry Isot. and Radio Generators.Dept, Faculty of Science Benha Univ. Atomic Energy Authority Dr. Shoukar T.M. Atwa Dr. A.A. El-Mohty Lect. of Physical Chemistry Pro Ass it. Prof.of Radiochemistry Faculty of Science Benha Univ. Isot. and Radio Generators.Dept, Atomic Energy Authority 2010 آ ام اء درات آ إ اد- ١٣١و اآت ا د ا ر ــــد س ا ا واات ا – آ ا ارة ه ا ار ا آــ اــــ ــم – ــ ل در ا اء اـــــــــاف أ.د/ ا ا أ.د/ د اذ اء ا و ا أذ اــء اـــ آ ام - ـــ ا واات ا هــــ اـــ ارــــــــ د/ ر أ.م.د / أ ا رس اء ا أذ اــء اـــ آ ام - ـــ ا واات ا هــــ اـــ ارــــــــ ٢٠١٠ List of abbreviations Abbr. Referent CAT Chloramine-T H2O2 Hydrogen peroxide HPLC High performance liquid chromatography TLC Thin layer chromatography Temp. Temperature Conc. Concentration min Minute NCA No Carrier Added Rf Relative front Rt Retention time CNS Central nervous system Y-indole 4-[2-hydroxy-3- (isopentylamino)propoxy] indole Epidepride N-[(1-ethyl-2- pyrrolidyl)methyl]-2,3- dimethoxy-5-(tributylstannyl) benzamide -

22Sra 226Ra and 222Rn in ' SOUTH CAROLINA GROUND WATER
Report No. 95 WRRI 22sRa 226Ra AND 222Rn IN ' SOUTH CAROLINA GROUND WATER: MEASUR~MEN-T TECHNIQUES AND ISOTOPE RELATIONSHIPS GlANNll\li FCUN~On o;: t,GR!CUL.TU~~tcoNOMlC2' ~~J.i\2Y J\li·iU ('lJ ~C 4· 1982 WATER RESOURCES RESEARCH INSTITUTE CLEMSON UNIVERSITY Clemson, .South Carolina JANUARY 1982 Water Resources Research Institute Clemson University Clemson, South Carolina 29631 ! 228Ra, 226Ra AND 222 Rn IN SOUTH CAROLINA GROUND WATER: MEASUREMENT TECHNIQUES AND ISOTOPE RELATIONSHIPS by Jacqueline Michel, Philip T. King and Willard S. Moore Department of Geology University of South Carolina Columbia, South Carolina 29208 The work upon which this puhlication is based was supported in part by funds provided by the Office of Water Research and Technology, project !!' No. OWRT-B-127-SC, U.S. Department of the Interior, fvashington, D. C., as authorized by the Water Research and Development Act of 1978 (PL95-467). r Project agreement No. 14-34-0001-9158 Period of Investigation: October 1979 - September 1980 Clemson University Water Resources Research Institute Technical Report No. 95 Contents of this publication do not necessarily reflect the views and policies of the Office of Water Research and Technology, U.S. Department of Interior, nor does mention of trade names or cormnercial products con stitute their endorsement or recommendation for use by the U.S. Goverrtment. ACKNOWLEDGEMENTS Much appreciation goes to Lewis Shaw, Rossie Stephens, Jim Ferguson and a large number of district personnel at the South Carolina Department of Health and Environmental Control who assisted us in field collection and provided much helpful information. Dr. -

Volatility of Radiopharmacy-Prepared Sodium Iodide-131 Capsules
RADIATION SAFETY Volatility of Radiopharmacy-Prepared Sodium Iodide-131 Capsules James M. Bright, Trenton T. Rees, Louis E. Baca and Richard L. Green Pharmacy Services, Syncor International Corporation, Albuquerque, New Mexico; and Quality and Regulatory Department, Syncor International Corporation, Woodland Hills, California studied thyroid physiology using 128I in 1937 (1,2). In January Objective: The aims of this study were to quantify the extent 1941, Hertz and Roberts were the first to administer radioiodine of volatilization from 131I-NaI therapeutic capsules prepared in 131I for the treatment of hyperthyroid patients (3). Today, almost a centralized radiopharmacy and to quantify the amount of 60 y later, radioiodine therapy with 131I remains the primary volatile 131I released from a dispensing vial containing a compounded 131I-NaI therapy capsule. therapeutic agent used in nuclear medicine, and its use is firmly Methods: Therapy capsules were prepared by injecting 131I established in the 2 diseases first treated: hyperthyroidism and oral solution into capsules containing anhydrous dibasic thyroid carcinoma. sodium phosphate. Volatilized activity was obtained by filter- Initially the use of 131I was restricted to the only pharmaceu- ing air drawn across samples that were placed open on the tical dosage form then available—liquid oral solution. While 131 bottom of a sample holder cup. Volatile I was captured by liquid radioiodine proved to be beneficial to the patients to filtering it through 3 triethylenediamine-impregnated carbon whom it was administered, the frequency of contamination and cartridge filters, arranged in series. To quantify the amount of thyroid uptake activity in nuclear medicine personnel who volatile 131I released from a dispensing vial during a simulated patient administration, a vial containing a compounded 131I handled the material was noted with increasing alarm (4–7). -
![小型飛翔体/海外 [Format 2] Technical Catalog Category](https://docslib.b-cdn.net/cover/2534/format-2-technical-catalog-category-112534.webp)
小型飛翔体/海外 [Format 2] Technical Catalog Category
小型飛翔体/海外 [Format 2] Technical Catalog Category Airborne contamination sensor Title Depth Evaluation of Entrained Products (DEEP) Proposed by Create Technologies Ltd & Costain Group PLC 1.DEEP is a sensor analysis software for analysing contamination. DEEP can distinguish between surface contamination and internal / absorbed contamination. The software measures contamination depth by analysing distortions in the gamma spectrum. The method can be applied to data gathered using any spectrometer. Because DEEP provides a means of discriminating surface contamination from other radiation sources, DEEP can be used to provide an estimate of surface contamination without physical sampling. DEEP is a real-time method which enables the user to generate a large number of rapid contamination assessments- this data is complementary to physical samples, providing a sound basis for extrapolation from point samples. It also helps identify anomalies enabling targeted sampling startegies. DEEP is compatible with small airborne spectrometer/ processor combinations, such as that proposed by the ARM-U project – please refer to the ARM-U proposal for more details of the air vehicle. Figure 1: DEEP system core components are small, light, low power and can be integrated via USB, serial or Ethernet interfaces. 小型飛翔体/海外 Figure 2: DEEP prototype software 2.Past experience (plants in Japan, overseas plant, applications in other industries, etc) Create technologies is a specialist R&D firm with a focus on imaging and sensing in the nuclear industry. Createc has developed and delivered several novel nuclear technologies, including the N-Visage gamma camera system. Costainis a leading UK construction and civil engineering firm with almost 150 years of history. -

Calcined Bone Provides a Reliable Substrate for Strontium Isotope Ratios As Shown by an Enrichment Experiment
Research Article Received: 8 August 2014 Revised: 15 October 2014 Accepted: 16 October 2014 Published online in Wiley Online Library Rapid Commun. Mass Spectrom. 2015, 29, 107–114 (wileyonlinelibrary.com) DOI: 10.1002/rcm.7078 Calcined bone provides a reliable substrate for strontium isotope ratios as shown by an enrichment experiment Christophe Snoeck1*, Julia Lee-Thorp1,RickSchulting1,JeroendeJong2, Wendy Debouge2 and Nadine Mattielli2 1Research Laboratory for Archaeology and the History of Art, University of Oxford, Dyson Perrins Building, South Parks Rd, Oxford OX1 3QY, UK 2G-Time Laboratory, Université Libre de Bruxelles, CP 160/02, 50, Avenue F.D. Roosevelt, B-1050 Brussels, Belgium RATIONALE: Strontium isotopes (87Sr/86Sr) are used in archaeological and forensic science as markers of residence or mobility because they reflect the local geological substrate. Currently, tooth enamel is considered to be the most reliable tissue, but it rarely survives heating so that in cremations only calcined bone fragments survive. We set out to test the proposition that calcined bone might prove resistant to diagenesis, given its relatively high crystallinity, as the ability to measure in vivo 87Sr/86Sr from calcined bone would greatly extend application to places and periods in which cremation was the dominant mortuary practice, or where unburned bone and enamel do not survive. METHODS: Tooth enamel and calcined bone samples were exposed to a 87Sr-spiked solution for up to 1 year. Samples were removed after various intervals, and attempts were made to remove the contamination using acetic acid washes and ultrasonication. 87Sr/86Sr was measured before and after pre-treatment on a Nu Plasma multi-collector induced coupled plasma mass spectrometer using NBS987 as a standard. -

Potassium-40 What Is It? Potassium Is a Soft, Silver-White Metal
Human Health Fact Sheet ANL, October 2001 Potassium-40 What Is It? Potassium is a soft, silver-white metal. An important constituent of soil, it is widely distributed in nature and is present in all Symbol: K(-40) plant and animal tissues. Potassium-40 is a naturally occurring Atomic Number: 19 radioactive isotope of potassium. (An isotope is a different form of an (protons in nucleus) element that has the same number of protons in the nucleus but a different number of neutrons.) Two stable (nonradioactive) isotopes of Atomic Weight: 39 potassium exist, potassium-39 and potassium-41. Potassium-39 (naturally occurring) comprises most (about 93%) of naturally occurring potassium, and potassium-41 accounts for essentially all the rest. Radioactive postassium-40 comprises a very small fraction (about 0.012%) of naturally occurring potassium. Several radioactive isotopes of potassium exist in addition to potassium-40. These isotopes all have half- lives of less than one day Radioactive Properties of Potassium-40 so they are not Natural Specific Radiation Energy (MeV) Half-Life Decay of concern for Isotope Abundance Activity (yr) Mode Alpha Beta Gamma Department of (%) (Ci/g) (α) (β) (γ) Energy (DOE) K-40 1.3 billion 0.012 0.0000071 β, EC - 0.52 0.16 environmental management EC = electron capture, Ci = curie, g = gram, and MeV = million electron volts; a dash means sites such as that the entry is not applicable. (See the companion fact sheet on Radioactive Properties, Hanford. The Internal Distribution, and Risk Coefficients for explanation of terms and interpretation of radiation energies.) Potassium-40 decays by both emitting a beta particle (89%) and electron half-life of capture (11%). -

Measurements of Fission Cross Sections for the Isotopes Relevant to the Thorium Fuel Cycle
EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH CERN/INTC 2001-025 INTC/P-145 08 Aug 2001 Measurements of Fission Cross Sections for the Isotopes relevant to the Thorium Fuel Cycle The n_TOF Collaboration ABBONDANNO U.20, ANDRIAMONJE S.10, ANDRZEJEWSKI J.24, ANGELOPOULOS A.12, ASSIMAKOPOULOS P.15, BACRI C-O.8, BADUREK G.1, BAUMANN P.9, BEER H.11, BENLLIURE J.32, BERTHIER B.8, BONDARENKO I.26, BOS A.J.J.36, BUSTREO N.19, CALVINO F.33, CANO-OTT D.28, CAPOTE R.31, CARLSON P.34, CHARPAK G.5, CHAUVIN N.8, CENNINI P.5, CHEPEL V.25, COLONNA N.20, CORTES G.33, CORTINA D.32, CORVI F.23, DAMIANOGLOU D.16, DAVID S.8, DIMOVASILI E.12, DOMINGO C.29, DOROSHENKO A.27, DURAN ESCRIBANO I.32, ELEFTHERIADIS C.16, EMBID M.28, FERRANT L.8, FERRARI A.5, FERREIRA–MARQUES R.25, FRAIS–KOELBL H.3, FURMAN W.26, FURSOV B.27, GARZON J.A.32, GIOMATARIS I.10, GLEDENOV Y.26, GONZALEZ–ROMERO E.28, GOVERDOVSKI A.27, GRAMEGNA F.19, GRIESMAYER E.3, GUNSING F.10, HAIGHT R.37, HEIL M.11, HOLLANDER P.36, IOANNIDES K.G.15, IOANNOU P.12, ISAEV S.27, JERICHA E.1, KADI Y.5, KAEPPELER F.11, KARADIMOS D.17, KARAMANIS D.15, KAYUKOV A.26, KAZAKOV L.27, KELIC A.9, KETLEROV V.27, KITIS G.16, KOEHLER P.E.38., KOPACH Y.26, KOSSIONIDES E.14, KROSHKINA I.27, LACOSTE V.5, LAMBOUDIS C.16, LEEB H.1, LEPRETRE A.10, LOPES M.25, LOZANO M.31, MARRONE S.20, MARTINEZ-VAL J. -

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements .