Morphea: Clinical Considerations and Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interstitial Granuloma Annulare Triggered by Lyme Disease
Volume 27 Number 5| May 2021 Dermatology Online Journal || Case Presentation 27(5):11 Interstitial granuloma annulare triggered by Lyme disease Jordan Hyde1 MD, Jose A Plaza1,2 MD, Jessica Kaffenberger1 MD Affiliations: 1Division of Dermatology, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA, 2Department of Pathology, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA Corresponding Author: Jessica Kaffenberger MD, Division of Dermatology, The Ohio State University Medical Wexner Medical Center, Suite 240, 540 Officenter Place, Columbus, OH 43230, Tel: 614-293-1707, Email: [email protected] been associated with a variety of systemic diseases Abstract including diabetes mellitus, malignancy, thyroid Granuloma annulare is a non-infectious disease, dyslipidemia, and infection [3,4]. granulomatous skin condition with multiple different associations. We present a case of a man in his 60s There are multiple histological variants of GA, with a three-week history of progressive targetoid including interstitial GA. The histopathology of plaques on his arms, legs, and trunk. Skin biopsy classic GA demonstrates a focal degeneration of demonstrated interstitial granuloma annulare. collagen surrounded by an inflammatory infiltrate Additional testing revealed IgM antibodies to Borrelia composed of lymphocytes and histiocytes. In a less burgdorferi on western blot suggesting interstitial common variant, interstitial GA, scattered histiocytes granuloma annulare was precipitated by the recent are seen -

2017 Oregon Dental Conference® Course Handout
2017 Oregon Dental Conference® Course Handout Nasser Said-Al-Naief, DDS, MS Course 8125: “The Mouth as The Body’s Mirror: Oral, Maxillofacial, and Head and Neck Manifestations of Systemic Disease” Thursday, April 6 2 pm - 3:30 pm 2/28/2017 The Mouth as The Body’s Mirror Oral Maxillofacial and Head and Neck Manifestation of Ulcerative Conditions of Allergic & Immunological Systemic Disease the Oro-Maxillofacial Diseases Region Nasser Said-Al-Naief, DDS, MS Professor & Chair, Oral Pathology and Radiology Director, OMFP Laboratory Oral manifestations of Office 503-494-8904// Direct: 503-494-0041 systemic diseases Oral Manifestations of Fax: 503-494-8905 Dermatological Diseases Cell: 1-205-215-5699 Common Oral [email protected] Conditions [email protected] OHSU School of Dentistry OHSU School of Medicine 2730 SW Moody Ave, CLSB 5N008 Portland, Oregon 97201 Recurrent aphthous stomatitis (RAS) Recurrent aphthous stomatitis (RAS) • Aphthous" comes from the Greek word "aphtha”- • Recurrence of one or more painful oral ulcers, in periods of days months. = ulcer • Usually begins in childhood or adolescence, • The most common oral mucosal disease in North • May decrease in frequency and severity by age America. (30+). • Affect 5% to 66% of the North American • Ulcers are confined to the lining (non-keratinized) population. mucosa: • * 60% of those affected are members of the • Buccal/labial mucosa, lateral/ventral tongue/floor of professional class. the mouth, soft palate/oropharyngeal mucosa • Etiopathogenesis: 1 2/28/2017 Etiology of RAU Recurrent Aphthous Stomatitis (RAS): Types: Minor; small size, shallow, regular, preceeded by prodrome, heal in 7-10 days Bacteria ( S. -

A Patient with Plaque Type Morphea Mimicking Systemic Lupus Erythematosus
CASE REPORT A Patient With Plaque Type Morphea Mimicking Systemic Lupus Erythematosus Wardhana1, EA Datau2 1 Department of Internal Medicine, Siloam International Hospitals. Karawaci, Indonesia. 2 Department of Internal Medicine, Prof. Dr. RD Kandou General Hospital & Sitti Maryam Islamic Hospital, Manado, North Sulawesi, Indonesia. Correspondence mail: Siloam Hospitals Group’s CEO Office, Siloam Hospital Lippo Village. 5th floor. Jl. Siloam No.6, Karawaci, Indonesia. email: [email protected] ABSTRAK Morfea merupakan penyakit jaringan penyambung yang jarang dengan gambaran utama berupa penebalan dermis tanpa disertai keterlibatan organ dalam. Penyakit ini juga dikenal sebagai bagian dari skleroderma terlokalisir. Berdasarkan gambaran klinis dan kedalaman jaringan yang terlibat, morfea dikelompokkan ke dalam beberapa bentuk dan sekitar dua pertiga orang dewasa dengan morfea mempunyai tipe plak. Produksi kolagen yang berlebihan oleh fibroblast merupakan penyebab kelainan pada morfea dan mekanisme terjadinya aktivitas fibroblast yang berlebihan ini masih belum diketahui, meskipun beberapa mekanisme pernah diajukan. Morfe tipe plak biasanya bersifat ringan dan dapat sembuh dengan sendirinya. Morfea tipe plak yang penampilan klinisnya menyerupai lupus eritematosus sistemik, misalnya meliputi alopesia dan ulkus mukosa di mulut, jarang dijumpai. Sebuah kasus morfea tipe plak pada wanita berusia 20 tahun dibahas. Pasien ini diobati dengan imunosupresan dan antioksidan local maupun sistemik. Kondisi paisen membaik tanpa disertai efek samping yang berarti. Kata kunci: morfea, tipe plak. ABSTRACT Morphea is an uncommon connective tissue disease with the most prominent feature being thickening or fibrosis of the dermal without internal organ involvement. It is also known as a part of localized scleroderma. Based on clinical presentation and depth of tissue involvement, morphea is classified into several forms, and about two thirds of adults with morphea have plaque type. -
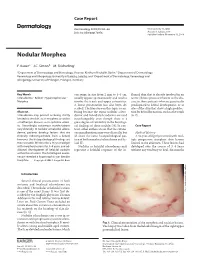
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable. -

S2 Table. List of Syntax for 96 Diseases
S2 Table. List of syntax for 96 diseases 'autoimmune gastritis'/exp OR 'acantholysis'/exp OR 'acantholysis' OR 'acute disseminated encephalomyelitis'/exp OR 'adem (acute disseminated encephalomyelitis)' OR 'acute disseminated encephalitis' OR 'acute disseminated encephalomyelitis' OR 'encephalitis postvaccinalis' OR 'encephalitis, post-vaccinal' OR 'encephalomyelitis, acute disseminated' OR 'post vaccinal encephalitis' OR 'post vaccination encephalitis' OR 'post-infectious encephalitis' OR 'post-infectious encephalomyelitis' OR 'postinfection encephalitis' OR 'postinfectious encephalitis' OR 'postinfectious encephalomyelitis' OR 'postvaccinal encephalitis' OR 'postvaccinal encephalopathy' OR 'postvaccination encephalitis' OR 'postvaccine encephalitis' OR 'postvaccinial encephalitis' OR 'postvaccinial encephalomyelitis' OR 'smallpox vaccination encephalitis' OR 'vaccinal encephalitis' OR 'vaccination encephalopathy' OR 'vaccination post vaccinial encephalitis' OR 'vaccinia encephalitis' OR 'addison disease'/exp OR 'addison disease' OR 'addison`s disease' OR 'addisons disease' OR 'addison biermer disease' OR 'adult onset still disease'/exp OR 'adult onset still disease' OR 'still`s disease, adult- onset' OR 'allergic glomerulonephritis'/exp OR 'allergic glomerulonephritis' OR 'glomerulonephritis, allergic' OR 'glomerulonephritis, poststreptococcal' OR 'post streptococcal glomerulonephritis' OR 'poststreptococcal glomerulonephritis' OR 'anca associated vasculitis'/exp OR 'anca associated vasculitis' OR 'anca vasculitis' OR 'anca-associated -

Distinct Autoimmune Syndromes in Morphea a Review of 245 Adult and Pediatric Cases
STUDY Distinct Autoimmune Syndromes in Morphea A Review of 245 Adult and Pediatric Cases Justin J. Leitenberger, BS; Rachael L. Cayce, BS; Robert W. Haley, MD; Beverley Adams-Huet, MS; Paul R. Bergstresser, MD; Heidi T. Jacobe, MD Objective: To determine the prevalence of extracuta- fected less frequently than expected. The prevalence of neous manifestations and autoimmunity in adult and pe- concomitant autoimmunity in the generalized subtype diatric patients with morphea. of morphea was statistically significantly greater than that found in all other subtypes combined (P=.01). Fre- Design: A retrospective review of 245 patients with quency of a family history of autoimmune disease showed morphea. a trend in favor of generalized and mixed subgroups. The linear subtype showed a significant association with neu- Setting: University of Texas Southwestern Medical Cen- rologic manifestations, while general systemic manifes- ter–affiliated institutions. tations were most common in the generalized subtype. Antinuclear antibody positivity was most frequent in Patients: Patients with clinical findings consistent with mixed and generalized subtypes. morphea. Conclusions: High prevalences of concomitant and fa- Main Outcome Measures: Prevalence of concomi- tant autoimmune diseases, prevalence of familial auto- milial autoimmune disease, systemic manifestations, and immune disease, prevalence of extracutaneous manifes- antinuclear antibody positivity in the generalized and pos- tations, and laboratory evidence of autoimmunity sibly mixed subtypes suggest that these are systemic au- (antinuclear antibody positivity). Secondary outcome toimmune syndromes and not skin-only phenomena. This measures included demographic features. has implications for the management and treatment of patients with morphea. Results: In this group, adults and children were af- fected nearly equally, and African Americans were af- Arch Dermatol. -

Postvaccination Morphea Profunda in a Child
Brief Reports 525 2. Dobru D, Seuchea N, Dorin M et al. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol 2004;13:237–240. 3. Nahm WK, Moise S, Eichenfield LF et al. Venous malfor- mations in blue rubber bleb nevus syndrome: variable onset of presentation. J Am Acad Dermatol 2004;5(Suppl): S101–S106. 4. Moodley M, Ramdial P. Blue rubber bleb nevus syndrome: case report and review of the literature. Pediatr 1993;92: 160–162. 5. Ertem D, Acar Y, Kotiloglu E et al. Blue rubber bleb nevus syndrome. Pediatr 2001;107:418–420. William Huang, M.D., M.P.H. Arthur Rhodes, M.D., M.P.H. Department of Dermatology, Rush University Medical Center, Chicago, Illinois Address correspondence to William Huang, M.D., M.P.H., Figure 2. H&E section (2·). 1653 West Congress Parkway, 220 Annex Building, Chicago, IL 60612, or e-mail: [email protected]. equally affected. Approximately 150 cases have been reported in the literature. The differential diagnosis Postvaccination Morphea Profunda in a Child includes familial cutaneous and mucosal venous mal- formation syndrome, familial glomangiomatosis, diffuse neonatal hemangiomatosis, and Maffuci syndrome (1). Abstract: We report a new case of postvaccination The most common site of visceral involvement is the morphea profunda (MP) in a child and discuss its dif- gastrointestinal tract, most commonly the small intes- ferent clinical presentations, prognosis, and therapy tine, documented by upper endoscopy, colonoscopy, or and its relationship with ‘‘solitary morphea profunda.’’ magnetic resonance imaging (MRI). Significant compli- A 2-year-old healthy girl presented with an induration of cations of gastrointestinal involvement include gastro- the anterior aspect of the left thigh of 9 months dura- intestinal hemorrhage, intussusception, volvulus, tion. -

Buffalo Medical Group, P.C. Robert E
Buffalo Medical Group, P.C. Robert E. Kalb, M.D. Phone: (716) 630-1102 Fax: (716) 633-6507 Department of Dermatology 325 Essjay Road Williamsville, New York 14221 2 FOOT- 1 HAND SYNDROME 2 foot - 1 hand syndrome is a superficial infection of the skin caused by the common athlete's foot fungus. It is quite common for people to have a minor amount of an athlete's foot condition. This would appear as slight scaling and/or itching between the toes. In addition, patients may have thickened toenails as part of the athlete's foot condition. Again the problem on the feet is very common and often patients are not even aware of it. In some patients, however, the athlete's foot fungus can spread to another area of the body. For some strange and unknown reason, it seems to affect only one hand. That is why the condition is called 2 foot - 1 hand syndrome. It is not clear why the problem develops in only one hand or why the right or left is involved in some patients. Fortunately there is very effective treatment to control this minor skin problem. If the problem with the superficial fungus infection is confined to the skin, then a short course of treatment with an oral antibiotic is all that is required. This antibiotic is very safe and normally clears the skin up fairly rapidly. It is often used with a topical cream to speed the healing process. If, however, the fingernails of the affected hand are also involved then a more prolonged course of the antibiotic will be necessary. -

Immunofluorescence in Dermatology
CONTINUING MEDICAL EDUCATION Immunofluorescence in dermatology Diya F. Mutasim, MD, and Brian B. Adams, MD Cincinnati, Ohio The accurate diagnosis of bullous and other immune diseases of the skin requires evaluation of clinical, histologic, and immunofluorescence findings. Immunofluorescence testing is invaluable in confirming a diagnosis that is suspected by clinical or histologic examination. This is especially true in subepidermal bullous diseases that often have overlap in the clinical and histologic findings. Direct immunofluorescence is performed on perilesional skin for patients with bullous diseases and lesional skin for patients with connective tissue diseases and vasculitis. (J Am Acad Dermatol 2001;45:803-22.) Learning objective: At the completion of this learning activity, participants should be familiar with the ideal method of obtaining immunofluorescence testing for the diagnosis of immune skin diseases and be aware of the value and limitations of immunofluorescence studies. mmunofluorescence has been used for 4 decades, both to investigate pathophysiology of Abbreviations used: skin disorders and to help physicians in the diag- I BMZ: basement membrane zone nosis of various cutaneous disorders, especially bul- BP: bullous pemphigoid lous diseases and connective tissue diseases. This CP: cicatricial pemphigoid article addresses the present status of immunofluo- DEJ: dermoepidermal junction rescence in dermatology. DH: dermatitis herpetiformis DIF: direct immunofluorescence DIAGNOSIS AND PATHOPHYSIOLOGY OF DLE: discoid lupus erythematosus BULLOUS DISEASES EBA: epidermolysis bullosa acquisita Great progress has been made during the past 5 HG: herpes gestationis decades in our understanding of the biology of the HSP: Henoch-Schönlein purpura ICS: intercellular space skin as it relates to bullous diseases. This has led to IIF: indirect immunofluorescence more accurate classification and diagnosis. -

Laser Therapy for the Treatment of Morphea: a Systematic Review of Literature
Journal of Clinical Medicine Review Laser Therapy for the Treatment of Morphea: A Systematic Review of Literature Paulina Szczepanik-Kułak * , Małgorzata Michalska-Jakubus and Dorota Krasowska Chair and Department of Dermatology, Venerology and Paediatric Dermatology, Medical University of Lublin, 20-081 Lublin, Poland; [email protected] (M.M.-J.); [email protected] (D.K.) * Correspondence: [email protected] Abstract: Morphea, also known as localized scleroderma (LoS), comprises a set of autoimmune sclerotic skin diseases. It is characterized by inflammation and limited thickening and induration of the skin; however, in some cases, deeper tissues might also be involved. Although morphea is not considered a life-threatening disease, the apparent cosmetic disfigurement, functional or psychosocial impairment affects multiple fields of patients’ quality of life. Therapy for LoS is often unsatisfactory with numerous treatments that have only limited effectiveness or considerable side effects. Due to the advances in the application of lasers and their possible beneficial effects, the aim of this study is to review the reported usage of laser in morphea. We present a systematic review of available literature, performed with MEDLINE, Cinahl, Central, Scopus, Web of Science, and Google Scholar databases. We identified a total of twenty relevant studies (MEDLINE n = 10, Cinahl n = 1, Central n = 0, Scopus n = 2, Web of Science n = 5, Google Scholar n = 2) using laser therapy for LoS. Eight studies were focused on the use of PDL, six on fractional lasers (CO2 and Er:YAG), four on excimer, and two on either alexandrite or Nd:YAG. Keywords: morphea; localized scleroderma; laser therapy Citation: Szczepanik-Kułak, P.; Michalska-Jakubus, M.; Krasowska, D. -

Morphea Affecting Bilateral Ankles
Henry Ford Health System Henry Ford Health System Scholarly Commons Case Reports Medical Education Research Forum 2020 5-2020 Morphea Affecting Bilateral Ankles Katherine Kloberdanz Henry Ford Health System, [email protected] Chris Olenech Henry Ford Health System, [email protected] Follow this and additional works at: https://scholarlycommons.henryford.com/merf2020caserpt Recommended Citation Kloberdanz, Katherine and Olenech, Chris, "Morphea Affecting Bilateral Ankles" (2020). Case Reports. 63. https://scholarlycommons.henryford.com/merf2020caserpt/63 This Poster is brought to you for free and open access by the Medical Education Research Forum 2020 at Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Case Reports by an authorized administrator of Henry Ford Health System Scholarly Commons. Morphea Affecting Bilateral Ankles Katherine Kloberdanz DPM, PGY-1 and Chris Olenech DPM Henry Ford Health System, Detroit, Michigan Introduction Images Results Morphea, also known as localized scleroderma, is an Over the course of two years, our patient’s morphea, which inflammatory disorder causing patches of hardened or discolored presented in a generalized pattern, flared into a more bullous skin and subcutaneous tissue and has an incidence of about 0.4- form. Her CellCept has been increased over time from 500 mg 2.7 per 100,000. The five main types of morphea include linear, TID to 1500 mg bid. Then in January 2020, the patient then plaque, generalized, deep, and bullous. More severe forms can began complaining that the skin hardening was spreading and lead to devastating functional and cosmetic impairment due to that she had more difficulty walking. She was admitted to the excessive collagen deposition in the deeper subcutaneous tissue. -

Necrobiotic Xanthogranuloma with Scleroderma
CONTINUING MEDICAL EDUCATION Necrobiotic Xanthogranuloma With Scleroderma Glenn G. Russo, MD GOAL To understand the presentation and treatment of necrobiotic xanthogranuloma (NXG) OBJECTIVES Upon completion of this activity, dermatologists and general practitioners should be able to: 1. Explain the laboratory and histopathology results in NXG. 2. Discuss the theoretical pathogenesis of NXG. 3. Describe the treatment options for NXG. CME Test on page 317. This article has been peer reviewed and Medicine is accredited by the ACCME to provide approved by Michael Fisher, MD, Professor of continuing medical education for physicians. Medicine, Albert Einstein College of Medicine. Albert Einstein College of Medicine designates Review date: November 2002. this educational activity for a maximum of 1.0 hour This activity has been planned and implemented in category 1 credit toward the AMA Physician’s in accordance with the Essential Areas and Policies Recognition Award. Each physician should claim of the Accreditation Council for Continuing Medical only those hours of credit that he/she actually spent Education through the joint sponsorship of Albert in the educational activity. Einstein College of Medicine and Quadrant This activity has been planned and produced in HealthCom, Inc. The Albert Einstein College of accordance with ACCME Essentials. Dr. Russo reports no conflict of interest. The author reports off-label use of the following medications: extracorporeal photophoresis, cyclophosphamide, methotrexate, nitrogen mustard, adrenocorticotropic hormone, azathioprine, radiation therapy, plasmapheresis, hydroxychloroquine, thalidomide, and etretinate. Dr. Fisher reports no conflict of interest. I report the case of a 68-year-old man who pre- exposure to cold temperatures. This is an interest- sented with necrobiotic xanthogranuloma (NXG) ing constellation of findings in this rare disorder with concomitant scleroderma, along with the pres- that raises questions concerning its pathogenesis.