Supplemental Tables Table S1. Expression Pattern of Genes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2013/0089535 A1 Yamashiro Et Al
US 2013 0089535A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0089535 A1 Yamashiro et al. (43) Pub. Date: Apr. 11, 2013 (54) AGENT FOR REDUCING ACETALDEHYDE Publication Classification NORAL CAVITY (51) Int. Cl. (75) Inventors: Kan Yamashiro, Kakamigahara-shi (JP); A68/66 (2006.01) Takahumi Koyama, Kakamigahara-shi A638/51 (2006.01) (JP) A61O 11/00 (2006.01) A638/44 (2006.01) Assignee: AMANOENZYME INC., Nagoya-shi (52) U.S. Cl. (73) CPC. A61K 8/66 (2013.01); A61K 38/44 (2013.01); (JP) A61 K38/51 (2013.01); A61O II/00 (2013.01) (21) Appl. No.: 13/703,451 USPC .......... 424/94.4; 424/94.5; 435/191: 435/232 (22) PCT Fled: Jun. 7, 2011 (57) ABSTRACT Disclosed herein is a novel enzymatic agent effective in (86) PCT NO.: PCT/UP2011/062991 reducing acetaldehyde in the oral cavity. It has been found S371 (c)(1), that an aldehyde dehydrogenase derived from a microorgan (2), (4) Date: Dec. 11, 2012 ism belonging to the genus Saccharomyces and a threonine aldolase derived from Escherichia coli are effective in reduc (30) Foreign Application Priority Data ing low concentrations of acetaldehyde. Therefore, an agent for reducing acetaldehyde in the oral cavity is provided, Jun. 19, 2010 (JP) ................................. 2010-140O26 which contains these enzymes as active ingredients. Patent Application Publication Apr. 11, 2013 Sheet 1 of 2 US 2013/0089535 A1 FIG 1) 10.5 1 0 9.9.5 8. 5 CONTROL TA AD (BSA) ENZYME Patent Application Publication Apr. 11, 2013 Sheet 2 of 2 US 2013/0089535 A1 FIG 2) 110 the CONTROL (BSA) 100 354. -

Development of a Phage Display Library for Discovery of Antigenic Brucella Peptides Jeffrey Williams Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2018 Development of a phage display library for discovery of antigenic Brucella peptides Jeffrey Williams Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Microbiology Commons Recommended Citation Williams, Jeffrey, "Development of a phage display library for discovery of antigenic Brucella peptides" (2018). Graduate Theses and Dissertations. 16896. https://lib.dr.iastate.edu/etd/16896 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Development of a phage display library for discovery of antigenic Brucella peptides by Jeffrey Williams A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Microbiology Program of Study Committee: Bryan H. Bellaire, Major Professor Steven Olsen Steven Carlson The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this thesis. The Graduate College will ensure this thesis is globally accessible and will not permit alterations after a degree is conferred. Iowa State University -

Characterization of the Scavenger Cell Proteome in Mouse and Rat Liver
Biol. Chem. 2021; 402(9): 1073–1085 Martha Paluschinski, Cheng Jun Jin, Natalia Qvartskhava, Boris Görg, Marianne Wammers, Judith Lang, Karl Lang, Gereon Poschmann, Kai Stühler and Dieter Häussinger* Characterization of the scavenger cell proteome in mouse and rat liver + https://doi.org/10.1515/hsz-2021-0123 The data suggest that the population of perivenous GS Received January 25, 2021; accepted July 4, 2021; scavenger cells is heterogeneous and not uniform as previ- published online July 30, 2021 ously suggested which may reflect a functional heterogeneity, possibly relevant for liver regeneration. Abstract: The structural-functional organization of ammonia and glutamine metabolism in the liver acinus involves highly Keywords: glutaminase; glutamine synthetase; liver specialized hepatocyte subpopulations like glutamine syn- zonation; proteomics; scavenger cells. thetase (GS) expressing perivenous hepatocytes (scavenger cells). However, this cell population has not yet been char- acterized extensively regarding expression of other genes and Introduction potential subpopulations. This was investigated in the present study by proteome profiling of periportal GS-negative and There is a sophisticated structural-functional organization in perivenous GS-expressing hepatocytes from mouse and rat. the liver acinus with regard to ammonium and glutamine Apart from established markers of GS+ hepatocytes such as metabolism (Frieg et al. 2021; Gebhardt and Mecke 1983; glutamate/aspartate transporter II (GLT1) or ammonium Häussinger 1983, 1990). Periportal hepatocytes express en- transporter Rh type B (RhBG), we identified novel scavenger zymes required for urea synthesis such as the rate-controlling cell-specific proteins like basal transcription factor 3 (BTF3) enzyme carbamoylphosphate synthetase 1 (CPS1) and liver- and heat-shock protein 25 (HSP25). -

Supplemental Methods
Supplemental Methods: Sample Collection Duplicate surface samples were collected from the Amazon River plume aboard the R/V Knorr in June 2010 (4 52.71’N, 51 21.59’W) during a period of high river discharge. The collection site (Station 10, 4° 52.71’N, 51° 21.59’W; S = 21.0; T = 29.6°C), located ~ 500 Km to the north of the Amazon River mouth, was characterized by the presence of coastal diatoms in the top 8 m of the water column. Sampling was conducted between 0700 and 0900 local time by gently impeller pumping (modified Rule 1800 submersible sump pump) surface water through 10 m of tygon tubing (3 cm) to the ship's deck where it then flowed through a 156 µm mesh into 20 L carboys. In the lab, cells were partitioned into two size fractions by sequential filtration (using a Masterflex peristaltic pump) of the pre-filtered seawater through a 2.0 µm pore-size, 142 mm diameter polycarbonate (PCTE) membrane filter (Sterlitech Corporation, Kent, CWA) and a 0.22 µm pore-size, 142 mm diameter Supor membrane filter (Pall, Port Washington, NY). Metagenomic and non-selective metatranscriptomic analyses were conducted on both pore-size filters; poly(A)-selected (eukaryote-dominated) metatranscriptomic analyses were conducted only on the larger pore-size filter (2.0 µm pore-size). All filters were immediately submerged in RNAlater (Applied Biosystems, Austin, TX) in sterile 50 mL conical tubes, incubated at room temperature overnight and then stored at -80oC until extraction. Filtration and stabilization of each sample was completed within 30 min of water collection. -

Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_001591825Cyc: Bacillus vietnamensis NBRC 101237 Cellular Overview Connections between pathways are omitted for legibility. Anamika Kothari sn-glycerol phosphate phosphate pro phosphate phosphate phosphate thiamine molybdate D-xylose D-ribose glutathione 3-phosphate D-mannitol L-cystine L-djenkolate lanthionine α,β-trehalose phosphate phosphate [+ 3 more] α,α-trehalose predicted predicted ABC ABC FliY ThiT XylF RbsB RS10935 UgpC TreP PutP RS10200 PstB PstB RS10385 RS03335 RS20030 RS19075 transporter transporter of molybdate of phosphate α,β-trehalose 6-phosphate L-cystine D-xylose D-ribose sn-glycerol D-mannitol phosphate phosphate thiamine glutathione α α phosphate phosphate phosphate phosphate L-djenkolate 3-phosphate , -trehalose 6-phosphate pro 1-phosphate lanthionine molybdate phosphate [+ 3 more] Metabolic Regulator Amino Acid Degradation Amine and Polyamine Biosynthesis Macromolecule Modification tRNA-uridine 2-thiolation Degradation ATP biosynthesis a mature peptidoglycan a nascent β an N-terminal- -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -
Supplementary Data TMAO Microbiome R1
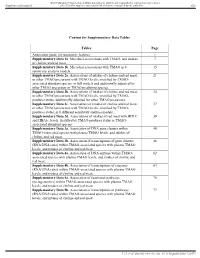
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Product Sheet Info
Master Clone List for NR-19279 ® Vibrio cholerae Gateway Clone Set, Recombinant in Escherichia coli, Plates 1-46 Catalog No. NR-19279 Table 1: Vibrio cholerae Gateway® Clones, Plate 1 (NR-19679) Clone ID Well ORF Locus ID Symbol Product Accession Position Length Number 174071 A02 367 VC2271 ribD riboflavin-specific deaminase NP_231902.1 174346 A03 336 VC1877 lpxK tetraacyldisaccharide 4`-kinase NP_231511.1 174354 A04 342 VC0953 holA DNA polymerase III, delta subunit NP_230600.1 174115 A05 388 VC2085 sucC succinyl-CoA synthase, beta subunit NP_231717.1 174310 A06 506 VC2400 murC UDP-N-acetylmuramate--alanine ligase NP_232030.1 174523 A07 132 VC0644 rbfA ribosome-binding factor A NP_230293.2 174632 A08 322 VC0681 ribF riboflavin kinase-FMN adenylyltransferase NP_230330.1 174930 A09 433 VC0720 phoR histidine protein kinase PhoR NP_230369.1 174953 A10 206 VC1178 conserved hypothetical protein NP_230823.1 174976 A11 213 VC2358 hypothetical protein NP_231988.1 174898 A12 369 VC0154 trmA tRNA (uracil-5-)-methyltransferase NP_229811.1 174059 B01 73 VC2098 hypothetical protein NP_231730.1 174075 B02 82 VC0561 rpsP ribosomal protein S16 NP_230212.1 174087 B03 378 VC1843 cydB-1 cytochrome d ubiquinol oxidase, subunit II NP_231477.1 174099 B04 383 VC1798 eha eha protein NP_231433.1 174294 B05 494 VC0763 GTP-binding protein NP_230412.1 174311 B06 314 VC2183 prsA ribose-phosphate pyrophosphokinase NP_231814.1 174603 B07 108 VC0675 thyA thymidylate synthase NP_230324.1 174474 B08 466 VC1297 asnS asparaginyl-tRNA synthetase NP_230942.2 174933 B09 198 -

Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Quang Ong Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_900114035Cyc: Amycolatopsis sacchari DSM 44468 Cellular Overview Connections between pathways are omitted for legibility. -

Enrichment, Isolation and Characterization of Phenazine-1-Carboxylic Acid (PCA)-Degrading Bacteria Under Aerobic and Anaerobic Conditions
Enrichment, isolation and characterization of phenazine-1-carboxylic acid (PCA)-degrading bacteria under aerobic and anaerobic conditions Miaomiao Zhang A thesis in fulfilment of the requirements for the degree of Doctor of Philosophy School of Civil and Environmental Engineering Faculty of Engineering September, 2018 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: ZHANG First name: Miaomiao Other name/s: Abbreviation for degree as given in the University calendar: PhD School: Civil and Environmental Engineering Faculty: Engineering Title: Enrichment, isolation and characterization of phenazine-1- carboxylic acid (PCA)-degrading bacteria under aerobic and anaerobic conditions Abstract Phenazines are a large class of nitrogen-containing aromatic heterocyclic compounds produced and secreted by bacteria from phylogenetically diverse taxa under aerobic and anaerobic conditions. Phenazine-1-carboxylic acid (PCA) is regarded as a ‘core’ phenazine because it is transformed to other phenazine derivatives. Due to their important roles in ecological fitness, biocontrol of plant pathogens, infection in cystic fibrosis and potential in anticancer treatments, understanding the fate of phenazine compounds is prudent. Only seven bacterial species are known to degrade phenazines and all of them are aerobic. Hence, the aim of this study is to enrich, isolate and characterize additional bacteria with the ability to degrade phenazines aerobically and anaerobically. In this study, the isolation of a PCA-degrading Rhodanobacter sp. PCA2 belonging to Grammaproteobacteria is reported. Characterization studies revealed that strain PCA2 is also capable of transforming other phenazines including phenazine, pyocyanin and 1-hydroxyphenazine. The sequencing, annotation and analysis of the genome of strain PCA2 revealed that genes (ubiD and the homolog of the MFORT_16269 gene) involved in PCA degradation were plasmid borne. -

Fermentation of Acetylene by an Obligate Anaerobe, Pelobacter Acetylenicus Sp
Archives of Arch Microbiol (1985) 142: 295- 301 Microbiology Springer-Verlag 1985 Fermentation of acetylene by an obligate anaerobe, Pelobacter acetylenicus sp. nov. * Bernhard Schink Fakult/it ffir Biologie, Universit/it Konstanz, Postfach 5560, D-7750 Konstanz, Federal Republic of Germany Abstract. Four strains of strictly anaerobic Gram-negative tion reactions (Schink 1985a). No significant anaerobic rod-shaped non-sporeforming bacteria were enriched and degradation could be observed with ethylene (ethene), the isolated from marine and freshwater sediments with acety- most simple unsaturated hydrocarbon (Schink 1985 a, b). lene (ethine) as sole source of carbon and energy. Acetylene, It was reported recently that also acetylene can be metab- acetoin, ethanolamine, choline, 1,2-propanediol, and glyc- olized in the absence of molecular oxygen (Watanabe and erol were the only substrates utilized for growth, the latter de Guzman 1980). Enrichment cultures with acetylene as two only in the presence of small amounts of acetate. Sub- sole carbon source were obtained in mineral media with strates were fermented by disproportionation to acetate and sulfate as electron acceptor, and acetate could be identified ethanol or the respective higher acids and alcohols. No as an intermediary metabolite (Culbertson et al. 1981). How- cytochromes were detectable; the guanine plus cytosine ever, these enrichment cultures were difficult to maintain, content of the DNA was 57.1 _+ 0.2 tool%. Alcohol dehy- and the acetylene-degrading bacteria could not be identified drogenase, aldehyde dehydrogenase, phosphate acetyl- (C. W. Culbertson and R. S. Oremland, Abstr. 3rd Int. transferase, and acetate kinase were found in high activities Syrup. -

Supplementary Informations SI2. Supplementary Table 1
Supplementary Informations SI2. Supplementary Table 1. M9, soil, and rhizosphere media composition. LB in Compound Name Exchange Reaction LB in soil LBin M9 rhizosphere H2O EX_cpd00001_e0 -15 -15 -10 O2 EX_cpd00007_e0 -15 -15 -10 Phosphate EX_cpd00009_e0 -15 -15 -10 CO2 EX_cpd00011_e0 -15 -15 0 Ammonia EX_cpd00013_e0 -7.5 -7.5 -10 L-glutamate EX_cpd00023_e0 0 -0.0283302 0 D-glucose EX_cpd00027_e0 -0.61972444 -0.04098397 0 Mn2 EX_cpd00030_e0 -15 -15 -10 Glycine EX_cpd00033_e0 -0.0068175 -0.00693094 0 Zn2 EX_cpd00034_e0 -15 -15 -10 L-alanine EX_cpd00035_e0 -0.02780553 -0.00823049 0 Succinate EX_cpd00036_e0 -0.0056245 -0.12240603 0 L-lysine EX_cpd00039_e0 0 -10 0 L-aspartate EX_cpd00041_e0 0 -0.03205557 0 Sulfate EX_cpd00048_e0 -15 -15 -10 L-arginine EX_cpd00051_e0 -0.0068175 -0.00948672 0 L-serine EX_cpd00054_e0 0 -0.01004986 0 Cu2+ EX_cpd00058_e0 -15 -15 -10 Ca2+ EX_cpd00063_e0 -15 -100 -10 L-ornithine EX_cpd00064_e0 -0.0068175 -0.00831712 0 H+ EX_cpd00067_e0 -15 -15 -10 L-tyrosine EX_cpd00069_e0 -0.0068175 -0.00233919 0 Sucrose EX_cpd00076_e0 0 -0.02049199 0 L-cysteine EX_cpd00084_e0 -0.0068175 0 0 Cl- EX_cpd00099_e0 -15 -15 -10 Glycerol EX_cpd00100_e0 0 0 -10 Biotin EX_cpd00104_e0 -15 -15 0 D-ribose EX_cpd00105_e0 -0.01862144 0 0 L-leucine EX_cpd00107_e0 -0.03596182 -0.00303228 0 D-galactose EX_cpd00108_e0 -0.25290619 -0.18317325 0 L-histidine EX_cpd00119_e0 -0.0068175 -0.00506825 0 L-proline EX_cpd00129_e0 -0.01102953 0 0 L-malate EX_cpd00130_e0 -0.03649016 -0.79413596 0 D-mannose EX_cpd00138_e0 -0.2540567 -0.05436649 0 Co2 EX_cpd00149_e0