Radioactivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Recollections and Reconstructions
@ ? Editor's Note: The Society's Nuclear Pioneer Citationfor 1977 goes not to an individual but to a distinguished group of individuals: those who took part in the epochal “ChicagoPile―experiment of Dec. 2, 1942, which produced thefirst successful atomicpile chain reaction. The 42 members ofthe Chicago Pilegroup are listed on page 591. One ofthose named, Harold M. Agnew, now Directorofthe Los Alamos Scient@flc Laboratory, is the Nuclear Pioneer Lecturerfor the 24th Annual Meeting. The guidingforce behind the Chicago Pile experiment was Enrico Fermi, recipient of the Nuclear Pioneer Citation of 1963. In the artick which follows, Laura Fermi recreates the excitement and mystery that surrounded the work ofher husband's research team in the early 1940s in Chicago. @. The FirstAtomic Pile: Recollections and Reconstructions by Laura Fermi @ . @ / - .-i@ ‘@:@ Enrico FermIat work, ca. 1942. As far as I can remember at the distance ofalmost 35 Perhaps not all husbands adhered as strictly as years, the first guests to arrive at our party on that Enrico to the rules of secrecy when talking to their beastly cold December night were the Zinns. Wally wives. He couldn't have been more tight-lipped. Once! and Jean. As they shook the snow off their coats, related some gossip to him: “Peoplesay that you stamping their feet on the floor, Wally turned to scientists at the Met Lab are seeking a cure for Enrico and said: “Congratulations!― I was surprised; cancer. .““Arewe?―he asked with his usual for Enrico had given me no hint that anything unusual imperturbable expression. -

MIT-NSE by the 1930S It Was Time to Discover Fission. We Knew About Isoto
Prelude to the Manhattan Project Michael V. Hynes – MIT-NSE By the 1930s it was time to discover fission. We knew about isotopes. The Curies and Becquerel had discovered radioactivity. Rutherford had discovered the nucleus. Chadwick had discovered the neutron. Einstein had discovered special relativity and the famous equation E=mc2. Bethe was even publishing about fusion as the source of energy for the Sun. So, it was time. By the 1930s a human drama was unfolding in Europe and in the Pacific – the rise of fascism. The events of this drama engulfed the world in war and created a need on all sides for weaponry that could defeat the enemy by whatever means. The intersection of scientific inevitability and war created the environment for the advent of nuclear weapons. Science was practiced was very differently in the 1920s and 1930s than today. In that era science was highly specialized and compartmentalized. If you were a metallurgist for example it was unlikely that you would have anything to do with a physicist – that all changed in the1940s. In 1933 Leo Szilard (Fig. 1), a Hungarian physicist who took refuge in London from Nazi Germany, read a paper by Rutherford that ridiculed the idea of getting energy from nuclear transmutations. Szilard realized that if you could find an element which is split by neutrons and which would emit two neutrons in the process, then a chain reaction could be started. This is the basic idea of a nuclear weapon -- to generate energy from the chain reaction. Szilard was a chemist by training and knew about the idea of a chemical chain reaction. -
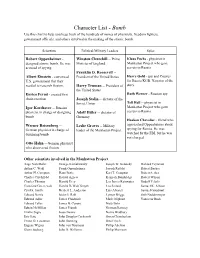
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Hitler's Uranium Club, the Secret Recordings at Farm Hall
HITLER’S URANIUM CLUB DER FARMHALLER NOBELPREIS-SONG (Melodie: Studio of seiner Reis) Detained since more than half a year Ein jeder weiss, das Unglueck kam Sind Hahn und wir in Farm Hall hier. Infolge splitting von Uran, Und fragt man wer is Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. The real reason nebenbei Die energy macht alles waermer. Ist weil we worked on nuclei. Only die Schweden werden aermer. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die nuclei waren fuer den Krieg Auf akademisches Geheiss Und fuer den allgemeinen Sieg. Kriegt Deutschland einen Nobel-Preis. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Wie ist das moeglich, fragt man sich, In Oxford Street, da lebt ein Wesen, The story seems wunderlich. Die wird das heut’ mit Thraenen lesen. Und fragt man, wer ist Schuld daran Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Die Feldherrn, Staatschefs, Zeitungsknaben, Es fehlte damals nur ein atom, Ihn everyday im Munde haben. Haett er gesagt: I marry you madam. Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. So ist die Antwort: Otto Hahn. Even the sweethearts in the world(s) Dies ist nur unsre-erste Feier, Sie nennen sich jetzt: “Atom-girls.” Ich glaub die Sache wird noch teuer, Und fragt man, wer ist Schuld daran, Und fragt man, wer ist Schuld daran, So ist die Antwort: Otto Hahn. -

Rutherford and Radon
Rediscovery of the Elements Rutherford and Radon U transmutation of the elements. He was to be (1877-1956) he developed the "transformation U acknowledged as the "leading explorer of the theory" which showed radioactivity was a vast, infinitely complex universe within the nuclear property. From thorium Rutherford atom, a universe that he was the first to pene- observed a gaseous radioactive product, which trate."3 he called "emanation"-he had discovered the Ernest Rutherford, the son of a flax farmer in element radon.' New Zealand, gained his undergraduate train- The British method of developing explicit ing in his native country. In 1895 he moved to descriptive models to explain nature was per- England, where he attended Cambridge Univ- fect for the advance of nuclear chemistry at this ersity; he was entering the scientific scene in moment in history; Lord Kelvin (William Europe just as radioactivity was discovered in Thomson, 1824-1907) said he could not reason 1896 by Henri Becquerel (1852-1908) of Paris. "without making a visualizable picture" of the His first research involved hertzian (radio) phenomenon he wanted to describe. J. J. James L. Marshal, beta Eta 1971, and waves, but he then moved on to the study of Thomson of the Cavendish Laboratory at Virginia R. Marshall, Beta Eta 2003, uranium rays with J. J. Thomson (1856-1940) at Cambridge was using the idea of charged cor- Department of Chemistry, University of Cambridge. Rutherford showed that these ion- puscles to explain cathode rays, and he viewed North Texas, Denton,TX 76203-5070, izing rays consisted of two main types, which the atom as a dynamic, moving mixture of pos- he called alpha and beta "for simplicity."2 itive and negative charges. -

From the Natural Transmutations of Uranium to Its Artificial Fission
O T T O H AH N From the natural transmutations of uranium to its artificial fission Nobel Lecture, December 13, 1946 The year 1946 marked a jubilee in the history of the chemical element, ura- nium. Fifty years earlier, in the spring of 1896, Henri Becquerel had discov- ered the remarkable radiation phenomena of this element, which were at that time grouped together under the name of radioactivity. For more than 100 years, uranium, discovered by W. H. Klaproth in 1789, had had a quiet existence as a somewhat rare but not particularly interesting element. After its inclusion in the Periodic System by D. Mendeleev and Lothar Meyer, it was distinguished from all the other elements in one partic- ular respect: it occupied the highest place in the table of the elements. As yet, however, that did not have any particular significance. We know today that it is just this position of uranium at the highest place of the then known chemical elements which gives it the important properties by which it is distinguished from all other elements. The echo of Becquerel’s fundamental observations on the radioactivity of uranium in scientific circles was at first fairly weak. Two years later, how- ever, they acquired an exceptional importance when the Curies succeeded in separating from uranium minerals two active substances, polonium and ra- dium, of which the latter appeared to be several million times stronger than the same weight of uranium. It was only a few years before the first surprising property of this "ra- diating" substance was explained. -

Nobel Prize Awards in Radiochemistry
Radiochim. Acta 100, 509–521 (2012) / DOI 10.1524/ract.2012.1953 © by Oldenbourg Wissenschaftsverlag, München Nobel Prize awards in Radiochemistry By J.-P. Adloff∗ University of Strasbourg, 63 Rue Saint Urbain, 67100 Strasbourg, France Dedicated to the memory of late Karl H. Lieser, Gerhard L. Stöcklin and Alfred P. Wolf with whom the author shared the editorial work of Radiochimica Acta from 1977 to 1995 (Received October 10, 2011; accepted in revised form January 19, 2012) (Published online March 26, 2012) Nobel Prize / Chemistry / Physics Summary. In 1996 the Editors of Radiochimica Acta brought out a special volume of the journal to celebrate the hundredth anniversary of the discovery of radioactivity [1]. On the occasion of the 50th anniversary of Radiochimica Acta, which follows closely upon the centenary of Marie Curie’s second Nobel Prize in 1911, the author has the privilege to informally review “Radiochemistry and Nobel Prize Awards”, including discoveries of radioelements and new fields in chemistry based on radiochemical methods. 1. The beginning The Nobel Prizes in Physics and Chemistry were estab- lished in 1901, six years after the discovery of radioactivity and three years after the discoveries of the elements polo- Fig. 1. Antoine Henri Becquerel (1852–1908). nium and radium. They are awarded by Kungliga Veten- skapakademien (the Royal Swedish Academy of Sciences) on the basis of proposals made by respective Committees rays when he thought the subject was exhausted. By the end on Physics and Chemistry, which receive recommendations of 1897 radioactivity was something of a dead horse: it was from Swedish and foreign scientists [2]. -

Chapter 4 Artificial Radioactive Isotopes
Irene and Frederic Joliot-Curie Chapter 4 Artificial Radioactive Isotopes The Discovery It was mentioned earlier that Irene Joliot-Curie and her husband Frederic Joliot-Curie was awarded the Nobel prize in Chemistry in 1935. The prize was given ”in recognition of their synthesis of new ra- dioactive elements” . Let us see more into the details of this experiment. Irène and Frederic Joliot-Curie had a large supply of polonium, after Irene`s parents. The polonium emitted alpha particles which they used to bombard different elements. In 1933 they used alpha particles and bombarded an aluminum plate. When they removed the α−particle source, it appeared that the aluminum plate emitted radiation with a half-life of approximately 3 minutes. The explanation was that the bombardment had resulted in a nuclear reaction. The α-particle penetrated the aluminum nucleus and changed it into phosphorus by emitting a neutron. The new phosphorus isotope was radioactive and was responsible for the observed radiation. Its designation is P-30. This nuclear reaction may be written as follows: 27 + α ⇒ 30 27 4 30 1 Al P + n or like this 13Al+ 2 He ⇒+ 15 P 0 n Decay of P-30 is: 30 30 15P⇒+ 14 Si positron Energy 3.24 MeV The neutron emitted can be observed as long as the bombardment takes place, but disappears im- mediately when the α-source is removed. However, the phosphorus isotope is radioactive. The decay mode is positron emission as shown above. The half-life is 2.5 minutes. 43 Irene and Fredric Joliot-Curie used their alpha bombardment technique on some other elements and found that it was possible to transform an element into another, with a higher number of protons in its nucleus. -

Preface and Acknowledgments
PREFACE AND ACKNOWLEDGMENTS It seems to me that I have always known of Lise Meitner. As a child I must have seen her picture in Life, or in The New York Times, or perhaps in the Aufbau, the German refugees' newspaper that my parents and grand mother often read. In America just after World War II, Lise Meitner was a celebrity: the tiny woman who barely escaped the Nazis, the physicist responsible for nuclear fission, "theJewish mother of the atomic bomb" although she was aJ ew by birth, not affiliation, and she had refused to work on the bomb. When I was six, the details didn't matter. To me, she was a hero, like Eleanor Roosevelt. I came back to Meitner thirty years later, in the 1970s, by way of a class I taught at California State University, Sacramento. Then, as now, I was on the chemistry faculty at Sacramento City College, a community college. At the university, I was known as the woman the all-male chemistry department did not want to hire; under such circumstances one becomes, and remains, a feminist. When the women's studies board asked me to put together a "Women in Science" course, I accepted right away, although at that moment I could think of only two: Marie Curie (of course) and Lise Meitner. So successful was feminist scholarship, however, that I was sure I would find more women in science and perhaps even begin to answer the question, Why so few? As it turns out, they were not so few. Throughout history, everywhere, women have been active in science and mathematics and medicine. -

The Women Behind the Periodic Table Brigitte Van Tiggelen and Annette Lykknes Spotlight Female Researchers Who Discovered Elements and Their Properties
COMMENT follow the Madelung rule. Although scan- differentiates it from calcium is a 3d one, even physicists and philosophers still need to step dium’s extra electron lies in its 3d orbital, though it is not the final electron to enter the in to comprehend the gestalt of the periodic experiments show that, when it is ionized, it atom as it builds up. table and its underlying physical explana- loses an electron from 4s first. This doesn’t In other words, the simple approach to tion. Experiments might shed new light, make sense in energetic terms — textbooks using the aufbau principle and the Made- too, such as the 2017 finding that helium say that 4s should have lower energy than 3d. lung rule remains valid for the periodic table can form the compound Na2He at very high Again, this problem has largely been swept viewed as a whole. It only breaks down when pressures11. The greatest icon in chemistry under the rug by researchers and educators. considering one specific atom and its occu- deserves such attention. ■ Schwarz used precise experimental pation of orbitals and ionization energies. spectral data to argue that scandium’s 3d The challenge of trying to derive the Eric Scerri is a historian and philosopher orbitals are, in fact, occupied before its 4s Madelung rule is back on. of chemistry at the University of California, orbital. Most people, other than atomic Los Angeles, California, USA. spectroscopists, had not realized this THEORIES STILL NEEDED e-mail: [email protected] before. Chemistry educators still described This knowledge about electron orbitals does 1.