A Monograph on the Protozoa of the Large Intestine of the Horse Ta-Shih Hsiung Iowa State College
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

The Life Cycle of Trypanosoma Cruzi
Tyler et al. THE LIFE CYCLE OF TRYPANOSOMA CRUZI K. M. Tyler, C. L. Olson and D. M. Engman Departments of Microbiology-Immunology and Pathology Feinberg Medical School of Northwestern University, Chicago, IL 60611 ABSTRACT Since the discovery of Trypanosoma cruzi as the parasite that causes Chagas disease, nearly a century ago, the details of the organism's life cycle have fascinated scientists. T. cruzi is a single-celled eukaryote with a complex life cycle alternating between reduviid bug vectors and vertebrate hosts. It is able to adapt via the process of cellular differentiation to replicate within the diverse environments represented of the insect's gut and host cell cytoplasm. These adaptive transformations take the form of coordinated changes in morphology, metabolism and cell cycle regulation. Different life cycle stages of T. cruzi show dramatically different protein and RNA profiles, which are the end result of unusual mechanisms for regulating gene expression. In recent years, new molecular techniques have been brought to bear on the life cycle dramatically increasing our knowledge of the strategies employed by the parasite to ensure its continued survival. INTRODUCTION Chagas disease The etiologic agent of the chronic and often fatal Chagas disease is the American trypanosome, Trypanosoma cruzi , a flagellated protozoan of the order Kinetoplastida. The survival of T. cruzi is dependent on the successful transmission between, and the colonization of, two radically different environments: the midgut of the reduviid bug vector and the cytoplasm of the mammalian host cell. As is true of all infections, interruption of the pathogen's life cycle will lead to eradication of the disease. -

A PRELIMINARY NOTE on TETRAMITUS, a STAGE in the LIFE CYCLE of a COPROZOIC AMOEBA by MARTHA BUNTING
294 Z6OL6G Y.- M. BUNTING PROC. N. A. S. A PRELIMINARY NOTE ON TETRAMITUS, A STAGE IN THE LIFE CYCLE OF A COPROZOIC AMOEBA By MARTHA BUNTING DEPARTMENT OF ZOOLOGY, UNIVERSITY OF PENNSYLVANIA Communicated July 24, 1922 In 1920 a study of the Entamoeba of the rat was begun and in a culture on artificial medium a number of Coprozoic amoeba appeared, one of which exhibited a flagellate phase which seems to be identical with Tetramitus rostratus Perty.1 The appearance of flagellate phases in cultures of Coprozoic amoeba (Whitmore,2 etc.) as well as of soil amoeba (Kofoid,1 Wilson,4 etc.) has been recorded a number of times, but the flagellates which appeared were relatively simple in organization and very transitory in occurrence. Fur- thermore, some of the simpler flagellates have been observed (Pascher5) to lose their flagella and become amoeboid. In the case here described, however, there is a transformation of a simple amoeba into a relatively complex flagellate which multiplies for several days then returns to the amoeboid condition and becomes encysted. Tetramitus rostratus has been studied by a number of investigators since its discovery in 1852, by Perty,' yet no one has given a full account of its life history. The lack of cysts in previous accounts, mentioned by Dobell and O'Connor,6 is explained by the transformation into an amoeba before encystment. It is believed that this animal presents the extreme in transformations of this sort and serves to emphasize the close relationship between amoebae and flagellates, and the need for careful studies of life cycles, in pure cultures where possible, in investigations of coprozoic and other Protozoa. -
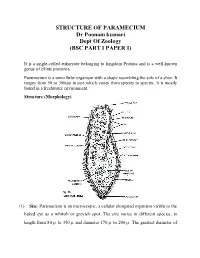
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

Identification of a Large Pre-Lysosomal Compartment in the Pathogenic Protozoon Trypanosoma Cruzi
Journal of Cell Science 102, 157-167 (1992) 157 Printed in Great Britain © The Company of Biologists Limited 1992 Identification of a large pre-lysosomal compartment in the pathogenic protozoon Trypanosoma cruzi MAURILIO J. SOARES1'2, THAIS SOUTO-PADR6N1 and WANDERLEY DE SOUZA1* 1 Departamento de Parasitologia e Bioflsica Celular, Institute/ de Biofisica Carlos Chagas Filho, UFRJ, 21949 Rio de Janeiro, RJ, Brazil 2Departamento de Ultraestrutura e Biologia Celular, Instituto Oswaldo Cruz, FIOCRUZ, 20001 Rio de Janeiro, RJ, Brazil •Author for correspondence Summary Epimastigote forms of the pathogenic parasite Trypano- from Lowicryl-embedded cells demonstrated that the soma cruzi were used to study the endocytic process in a reservosomes are acidic compartments (pH 6.0, as protozoon. These elongated unicellular organisms are shown using DAMP as a pH probe) with no acid highly polarized cells: endocytosis occurs only at the phosphatase or typical lysosome-associated membrane anterior region through the cytostome and the flagellar proteins (LAMP 1, LAMP 2 and lgp 120), but rich in pocket membrane, areas of the plasma membrane where cysteine proteinase. These data suggest that the reservo- the cell cytoskeleton, formed by sub-peUicular micro- some is a pre-lysosomal compartment. Since cysteine tubules, is absent. When the cells were incubated at 4°C proteinase of T. cruzi contains no phosphorylated or 28°C with gold-labeled transferrin, fixed and pro- mannose residues and the cation-independent mannose cessed for routine transmission electron microscopy our 6-phosphate receptor could not be immunocytochemi- observations show that this ligand initially binds to the cally detected hi the reservosomes, it is possible that cytostome and the membrane lining the flagellar pocket lysosomal enzymes hi the epimastigote forms of T. -

Chapter 2 : Cell Structure and Cell Organisation Subtopic : Cell Organisation Unicellular a Single Cell Performs All the Basic
Chapter 2 : Cell Structure and Cell Organisation Subtopic : Cell Organisation Unicellular A single cell performs all the basic life process. Example: Amoeba s p., Paramecium sp., Multicellular An organism consists of more than one cell. Each group of cell spe cialized to carry our life processes. Example: Homo sapien (human), animals and plants. It h as five levels of organisation 1. Cells: basic units of structure and function. Example: Red blood cells and xylem vessel cells. 2. Tissues: made up of cells with similar in structure and function. Example: Epithelial tissues and vascular tissues. 3. Organs: made up of tissues that perform a specific function. Example: Heart and flower. 4. System: two of more organs that perform a specific function. Example: Digestive system and root system. 5. Organisms: whole living thing that carry out all the basic life processes. Example: Human and durian tree. Cell Organisation (Unicellular) in Amoeba sp. (lives in freshwater ponds) and Pa ramecium sp. (lives in soil and moist area) 1. Cell structure · Amoeba sp.: plasma membrane, food vacuole, contractile vacuole, pseudopodium, nucleus, ectoplasma, endoplasm. · Paramecium sp.: food vacuole, posterior contractile vacuole, cytostome, gullet, oral groove, cilia, macronucleus, micronucleus, anterior contractile vacuole. 2. Locomotion · Amoeba sp.: Pseudopodium (false foot) helps it to move forward slowly and it is known as amoeboid movement. · Paramecium sp.: Hair-like cilia to beat against water. It beats its cilia backwa rds diagonally (swim forward) and it rotates on its axis. It beats its cilia forward (swim backwards). 3. Feeding 1 · Amoeba sp.: Omnivore. Eat bacteria, plant cells, algae and other microscopic org anisms. -

Four Distinct Pathways of Hemoglobin Uptake in the Malaria Parasite Plasmodium Falciparum
Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum David A. Elliott*†, Michael T. McIntosh‡, H. Dean Hosgood III§, Shuo Chen*, Gina Zhang*, Pavlina Baevova¶, and Keith A. Joiner* *Department of Cell Biology and Anatomy, University of Arizona College of Medicine, Tucson, AZ 85724; and Departments of ‡Internal Medicine, §Epidemiology and Public Health, and ¶Laboratory Medicine, Yale University School of Medicine, New Haven, CT 06520 Edited by Anthony A. James, University of California, Irvine, CA, and approved December 20, 2007 (received for review November 21, 2007) During the bloodstage of malaria infection, the parasite internalizes parasite itself has little defined internal organization. Thus, in any and degrades massive amounts of hemoglobin from the host red particular two-dimensional image of the parasite or the host cell, it blood cell. Using serial thin-section electron microscopy and three- is nearly impossible to determine the position or orientation of dimensional reconstruction, we demonstrate four independent, but anatomical features (7, 8). It is likewise difficult to relate different partially overlapping, hemoglobin-uptake processes distinguishable images to one another to map the morphologic and temporal temporally, morphologically, and pharmacologically. Early ring-stage sequence of hemoglobin uptake and delivery to the FV. For parasites undergo a profound morphological transformation in which example, although tubular hemoglobin-containing structures, when they fold, like a cup, onto themselves and in so doing take a large first viewed in cross section, cannot be distinguished from spherical gulp of host cell cytoplasm. This event, which we term the ‘‘Big Gulp,’’ vesicles, the literature is dominated by the assumption that discrete appears to be independent of actin polymerization and marks the first spherical vesicles mediate hemoglobin transport. -

"Plasmodium". In: Encyclopedia of Life Sciences (ELS)
Plasmodium Advanced article Lawrence H Bannister, King’s College London, London, UK Article Contents . Introduction and Description of Plasmodium Irwin W Sherman, University of California, Riverside, California, USA . Plasmodium Hosts Based in part on the previous version of this Encyclopedia of Life Sciences . Life Cycle (ELS) article, Plasmodium by Irwin W Sherman. Asexual Blood Stages . Intracellular Asexual Blood Parasite Stages . Sexual Stages . Mosquito Asexual Stages . Pre-erythrocytic Stages . Metabolism . The Plasmodium Genome . Motility . Recent History of Plasmodium Research . Evolution of Plasmodium . Conclusion Online posting date: 15th December 2009 Plasmodium is a genus of parasitic protozoa which infect Introduction and Description of erythrocytes of vertebrates and cause malaria. Their life cycle alternates between mosquito and vertebrate hosts. Plasmodium Parasites enter the bloodstream after a mosquito bite, Parasites of the genus Plasmodium are protozoans which and multiply sequentially within liver cells and erythro- invade and multiply within erythrocytes of vertebrates, and cytes before becoming male or female sexual forms. When are transmitted by mosquitoes. The motile invasive stages ingested by a mosquito, these fuse, then the parasite (merozoite, ookinete and sporozoite) are elongate, uni- multiplies again to form more invasive stages which are nucleate cells able to enter cells or pass through tissues, transmitted back in the insect’s saliva to a vertebrate. All using specialized secretory and locomotory organelles. -

The Cytostome–Cytopharynx Complex of Trypanosoma Cruzi Epimastigotes Disassembles During Cell Division Carolina De L
© 2017. Published by The Company of Biologists Ltd | Journal of Cell Science (2017) 130, 164-176 doi:10.1242/jcs.187419 SPECIAL ISSUE: 3D CELL BIOLOGY RESEARCH ARTICLE The cytostome–cytopharynx complex of Trypanosoma cruzi epimastigotes disassembles during cell division Carolina de L. Alcantara1,2, Juliana C. Vidal1,2, Wanderley de Souza1,2 and Narcisa L. Cunha-e-Silva1,2,* ABSTRACT underneath the cytostome membrane to the posterior of the cell, and The cytostome–cytopharynx complex is the main site for endocytosis four microtubules that run from staggered positions underneath the in epimastigotes of Trypanosoma cruzi. It consists of an opening at flagellar pocket membrane to the cytopharynx, following the path of the plasma membrane surface – the cytostome – followed by a the preoral ridge, a specialized membrane domain found between the membrane invagination – the cytopharynx. In G1/S cells, this flagellar pocket opening and the cytostome. Our tomography data structure is associated with two specific sets of microtubules, a show that the cytopharynx microtubule quartet is clearly distinct from quartet and a triplet. Here, we used electron microscopy and electron the microtubule quartet typically associated with the flagellum tomography to build 3D models of the complex at different stages of attachment zone in trypanosomatids (this latter quartet is denoted the cell cycle. The cytostome–cytopharynx is absent in late G2 and M MtQ) (Taylor and Godfrey, 1969; Vickerman, 1969; Lacomble et al., – phase cells, whereas early G2 cells have either a short cytopharynx 2009). These cytostome cytopharynx microtubules accompany the ‘ ’ or no visible complex, with numerous vesicles aligned to the cytopharynx along its path, in a typical gutter arrangement that cytostome–cytopharynx microtubules. -

Morphology, Ultrastructure, Genomics, and Phylogeny of Euplotes Vanleeuwenhoeki Sp
www.nature.com/scientificreports OPEN Morphology, ultrastructure, genomics, and phylogeny of Euplotes vanleeuwenhoeki sp. nov. and its ultra‑reduced endosymbiont “Candidatus Pinguicoccus supinus” sp. nov. Valentina Serra1,7, Leandro Gammuto1,7, Venkatamahesh Nitla1, Michele Castelli2,3, Olivia Lanzoni1, Davide Sassera3, Claudio Bandi2, Bhagavatula Venkata Sandeep4, Franco Verni1, Letizia Modeo1,5,6* & Giulio Petroni1,5,6* Taxonomy is the science of defning and naming groups of biological organisms based on shared characteristics and, more recently, on evolutionary relationships. With the birth of novel genomics/ bioinformatics techniques and the increasing interest in microbiome studies, a further advance of taxonomic discipline appears not only possible but highly desirable. The present work proposes a new approach to modern taxonomy, consisting in the inclusion of novel descriptors in the organism characterization: (1) the presence of associated microorganisms (e.g.: symbionts, microbiome), (2) the mitochondrial genome of the host, (3) the symbiont genome. This approach aims to provide a deeper comprehension of the evolutionary/ecological dimensions of organisms since their very frst description. Particularly interesting, are those complexes formed by the host plus associated microorganisms, that in the present study we refer to as “holobionts”. We illustrate this approach through the description of the ciliate Euplotes vanleeuwenhoeki sp. nov. and its bacterial endosymbiont “Candidatus Pinguicoccus supinus” gen. nov., sp. nov. The endosymbiont possesses an extremely reduced genome (~ 163 kbp); intriguingly, this suggests a high integration between host and symbiont. Taxonomy is the science of defning and naming groups of biological organisms based on shared characteristics and, more recently, based on evolutionary relationships. Classical taxonomy was exclusively based on morpho- logical-comparative techniques requiring a very high specialization on specifc taxa. -

Topic: Nutrition in Protozoa. B.Sc Part-I, Zoology (Hons
Topic: Nutrition in Protozoa. B.Sc Part-I, Zoology (Hons. and Sub), Paper-I (Group A), Lecture No. 10 By Dr. Priyanka Lal Assistant Professor Department of Zoology Women’s College Samastipur. Nutrition in Protozoa: Nutrition is a process by which the individuals obtain nourishment. It includes ingestion, digestion, absorption and digestion. The nutrition of protozoa is manifested by following ways: A. Holozoic or Zootrophic or Heterotrophic nutrition, B. Holotrophic or Autotrophic or Phytotrophic nutrition, C. Saprozoic or Saprophytic nutrition, D. Parasitic nutrition and E. Myxotrophic nutrition. (A) Holozoic or Zootrophic or Heterotrophic: In this method animals and plants smaller than the body of the protozoa are used as food sources (Fig. 1). The method is ultimately associated with ingestion, digestion, assimilation and egestion. I. Ingestion: Most Sarcodina capture their food and take them inside the body through any part of the body. In Mastigophora and Ciliates food enters into the body through the cytostome. The lashing of flagella or cilia aids in bringing about the food particles to the cytostome. Food- getting is no problem to parasitic forms when they are inside the body of the host. In Sarcodina the following methods have been observed for the ingestion of food par- ticles: (a) Import: Dr. Priyanka lal Page 1 The food is taken inside the body upon contact with little or no movement of body parts. (b) Circumfluence: The food is surrounded on all sides by the cytoplasm and is engulfed. (c) Circumvallation: The amoeba forms pseudopodia round the food particle and ingests it. (d) Invagination: The ectoplasm of the amoeba, when comes in contact with the food particle, is invaginated or in pushed into the endoplasm as a tube. -

Recruitment of Erythrocyte Membrane Components by Apicomplexan Parasites Plasmodium Falciparum and Babesia Divergens”
Recruitment of erythrocyte membrane components by apicomplexan parasites Babesia divergens and Plasmodium falciparum DISSERTATION zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) Dem Fachbereich Biologie der Philipps-Universität Marburg vorgelegt von Preetish Gangopadhyay aus Kalkutta, Indien Marburg am der Lahn 2015 Vom Fachbereich Biologie der Philipps-Universität Marburg als Dissertation angenommen am: Erstgutachter: Prof. Dr. Klaus Lingelbach Zweitgutachter: Prof. Dr. Ralf Jacob Tag der Disputation am: “I slept and dreamt that life was joy. I awoke and saw that life was service. I acted and behold, service was joy” Rabindranath Tagore To my Ma, Baba, Didi, Sonai, Mom-mom and Ayan A major part of the results presented in the thesis have been published in: Repnik, U., Gangopadhyay P., S. Bietz, J. M. Przyborski, G. Griffiths and K. Lingelbach (2015). "The apicomplexan parasite Babesia divergens internalizes band 3, glycophorin A and spectrin during invasion of human red blood cells." Cell Microbiol. And has been prepared to be published in a review as Novel insights into red blood cell physiology using parasites as tools (manuscript in preparation) Stefan Baumeister, Preetish Gangopadhyay, Urska Repnik, Klaus Lingelbach Other publications Thavayogarajah, T., P. Gangopadhyay, S. Rahlfs, K. Becker, K. Lingelbach, J. M. Przyborski and A. A. Holder (2015). "Alternative Protein Secretion in the Malaria Parasite Plasmodium falciparum." PLoS One 10(4): e0125191 Table of Contents Abbreviations Summary Zusammenfassung 1