Microscopes4schools
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Morphological Re-Description of Ploeotia Pseudanisonema, and Phylogenetic Analysis of the Genus Ploeotia
A Morphological Re-Description of Ploeotia pseudanisonema, and Phylogenetic Analysis of the Genus Ploeotia by Andrew Buren Brooks (Under the direction of Mark A. Farmer) Abstract The genus Ploeotia represents a group of small, colorless, heterotrophic euglenids commonly found in shallow-water marine sediments. Species belonging to this genus have histori- cally been poorly described and studied. However, a Group I intron discovered within the small subunit ribosomal DNA gene of Ploeotia costata, makes P. costata the only euglena- zoan known to possess an actively splicing Group I intron. This intron contains conserved secondary structures that indicate monophyly with introns found in Stramenopiles and Ban- giales red algae. This project describes the internal and external morphology of a related species, Ploeotia pseudanisonema, using light and electron microscopy, and investigates the possibility of a Group I intron within P. pseudanisonema. Using recently obtained SSU rRNA sequences, we also examine the phylogeny of Ploeotia, and comment on the relationship of the genus Keelungia to Ploeotia. Index words: Ploeotia pseudanisonema, Ploeotia costata, Ploeotia vitrea, Keelungia pulex, Transmission electron microscopy, Scanning electron microscopy, Small subunit rRNA, Euglenozoan Phylogeny A Morphological Re-Description of Ploeotia pseudanisonema, and Phylogenetic Analysis of the Genus Ploeotia by Andrew Buren Brooks B.S., University of Alabama, 2009 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree Master of Science Athens, Georgia 2010 c 2014 Andrew Buren Brooks All Right Reserved A Morphological Re-Description of Ploeotia pseudanisonema, and Phylogenetic Analysis of the Genus Ploeotia by Andrew Buren Brooks Approved: Major Professor: Mark A. -

Volvox Barberi Flocks, Forming Near-Optimal, Two
bioRxiv preprint doi: https://doi.org/10.1101/279059; this version posted March 8, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Volvox barberi flocks, forming near-optimal, two-dimensional, polydisperse lattice packings Ravi Nicholas Balasubramanian1 1Harriton High School, 600 North Ithan Avenue, Bryn Mawr, PA 19010, USA Volvox barberi is a multicellular green alga forming spherical colonies of 10000-50000 differentiated somatic and germ cells. Here, I show that these colonies actively self-organize over minutes into “flocks" that can contain more than 100 colonies moving and rotating collectively for hours. The colonies in flocks form two-dimensional, irregular, \active crystals", with lattice angles and colony diameters both following log-normal distributions. Comparison with a dynamical simulation of soft spheres with diameters matched to the Volvox samples, and a weak long-range attractive force, show that the Volvox flocks achieve optimal random close-packing. A dye tracer in the Volvox medium revealed large hydrodynamic vortices generated by colony and flock rotations, providing a likely source of the forces leading to flocking and optimal packing. INTRODUCTION behavior (see, e.g., [8, 9] and references therein) but their interactions are often dominated by viscous forces (e.g. fluid drag) unlike larger organisms which are The remarkable multicellular green alga Volvox barberi dominated by inertial forces. Here, I show that V. [1] forms spherical colonies of 10,000 to 50,000 cells barberi colonies, which are themselves composed of many embedded in a glycol-protein based extra cellular matrix individual cells acting together, show collective behavior (ECM) and connected by cytoplasmic bridges that may at a higher level of organization. -

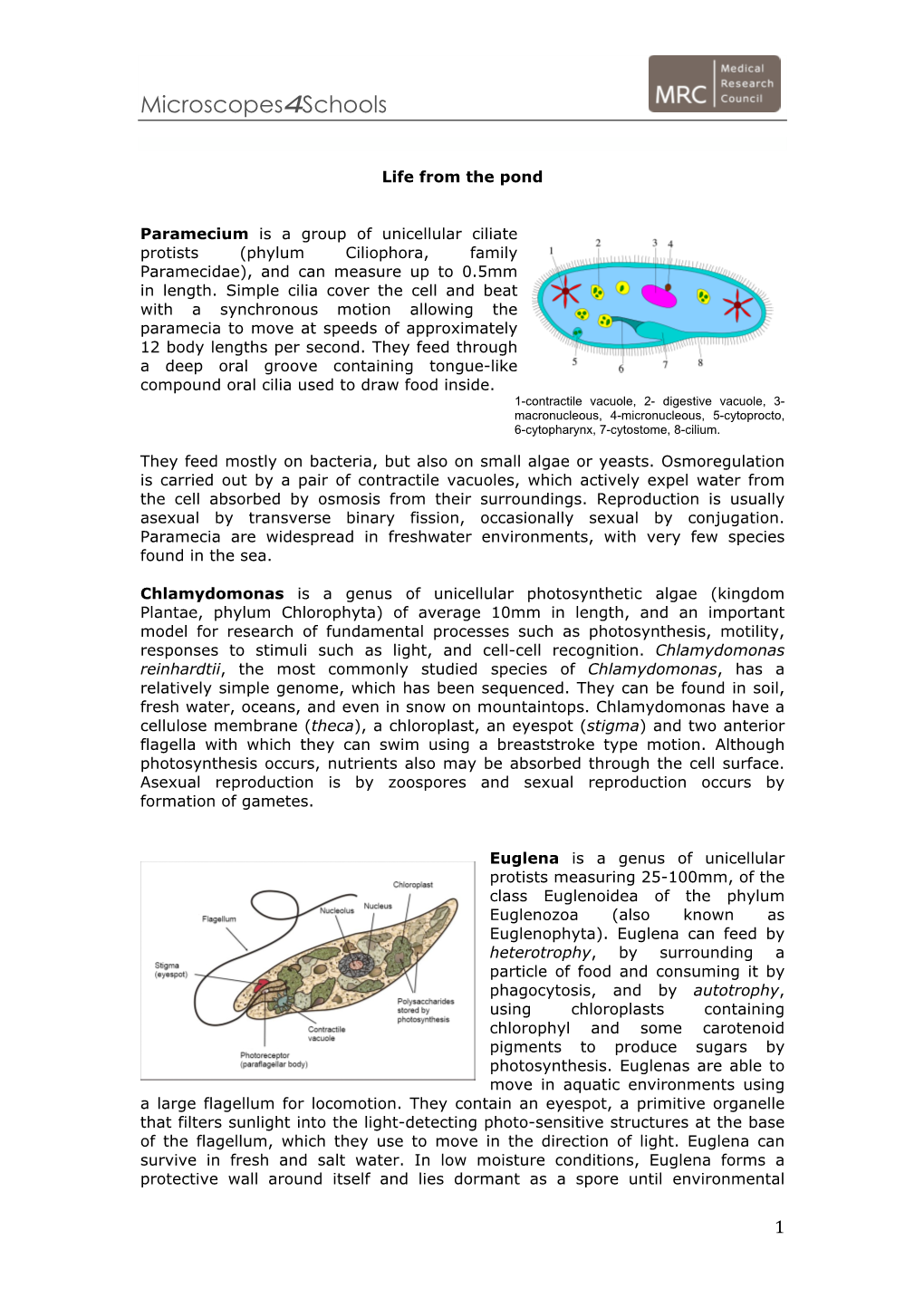
Microorganisms – Protists: Euglena
Microorganisms – Protists: Euglena Euglena are unicellular organisms classified into the Kingdom Protista, and the Phylum Euglenophyta. All euglena have chloroplasts and can make their own food by photosynthesis. They are not completely autotrophic though, euglena can also absorb food from their environment. Euglena usually live in quiet ponds or puddles. Euglena move by a flagellum (plural flagella), which is a long whip-like structure that acts like a little motor. The flagellum is located on the anterior (front) end, and twirls in such a way as to pull the cell through the water. It is attached at an inward pocket called the reservoir. Color and label the reservoir grey. Color and label the flagellum black. The Euglena is unique in that it is both heterotrophic (must consume food) and autotrophic (can make its own food). Chloroplasts within the euglena trap sunlight that is used for photosynthesis and can be seen as several rod-like structures throughout the cell. Color and label the chloroplasts green. Euglena also have an eyespot at the anterior end that detects light, it can be seen near the reservoir. This helps the euglena find bright areas to gather sunlight to make their food. Color and label the eyespot red. Euglena can also gain nutrients by absorbing them across their cell membrane, hence they become heterotrophic when light is not available, and they cannot photosynthesize. The euglena has a stiff pellicle outside the cell membrane that helps it keep its shape, though the pellicle is somewhat flexible, and some euglena can be observed scrunching up and moving in an inchworm type fashion. -

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -

Algae of the Genus Volvox (Chlorophyta) in Sub-Extreme Habitats T A.G
Short Communication T REPRO N DU The International Journal of Plant Reproductive Biology 12(2) July, 2020, pp.156-158 LA C P T I F V O E B Y T I DOI 10.14787/ijprb.2020 12.2. O E I L O C G O S I S T E S H Algae of the genus Volvox (Chlorophyta) in sub-extreme habitats T A.G. Desnitskiy Department of Embryology, Saint-Petersburg State University, Saint-Petersburg, 199034, Universitetskaya nab. 7/9, Russia e-mail: [email protected]; [email protected] Received: 18. 05. 2020; Revised: 08. 06. 2020; Accepted and Published online: 15. 06. 2020 ABSTRACT Literature data on the life of green colonial algae of the genus Volvox (Chlorophyta) in sub-extreme habitats (polar, sub-polar and mountain regions) are critically considered. Very few species (primarily homothallic Volvox aureus) are able to thrive in such conditions. Keywords : Geographical distribution, reproduction, sub-extreme habitats, Volvox. The genus Volvox Linnaeus (Volvocaceae, Chlorophyta) Peru (South America) at the elevation of more than five includes more than 20 species of freshwater flagellate algae thousand meters above sea level seems to be doubtful. The (Nozaki et al. 2015), providing an opportunity to study the illustration from this article (which focuses mainly on developmental mechanisms in a relatively simple system diatoms) shows a spherical colony with a diameter of about 14 consisting of two cellular types (somatic and reproductive). μm, consisting of several hundred very small cells (Fritz et al. Volvox carteri f. nagariensis Iyengar is a valuable model of 2015, p. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Superorganisms of the Protist Kingdom: a New Level of Biological Organization
Foundations of Science https://doi.org/10.1007/s10699-020-09688-8 Superorganisms of the Protist Kingdom: A New Level of Biological Organization Łukasz Lamża1 © The Author(s) 2020 Abstract The concept of superorganism has a mixed reputation in biology—for some it is a conveni- ent way of discussing supra-organismal levels of organization, and for others, little more than a poetic metaphor. Here, I show that a considerable step forward in the understand- ing of superorganisms results from a thorough review of the supra-organismal levels of organization now known to exist among the “unicellular” protists. Limiting the discussion to protists has enormous advantages: their bodies are very well studied and relatively sim- ple (as compared to humans or termites, two standard examples in most discussions about superorganisms), and they exhibit an enormous diversity of anatomies and lifestyles. This allows for unprecedented resolution in describing forms of supra-organismal organiza- tion. Here, four criteria are used to diferentiate loose, incidental associations of hosts with their microbiota from “actual” superorganisms: (1) obligatory character, (2) specifc spatial localization of microbiota, (3) presence of attachment structures and (4) signs of co-evolu- tion in phylogenetic analyses. Three groups—that have never before been described in the philosophical literature—merit special attention: Symbiontida (also called Postgaardea), Oxymonadida and Parabasalia. Specifcally, it is argued that in certain cases—for Bihos- pites bacati and Calkinsia aureus (symbiontids), Streblomastix strix (an oxymonad), Joe- nia annectens and Mixotricha paradoxa (parabasalids) and Kentrophoros (a ciliate)—it is fully appropriate to describe the whole protist-microbiota assocation as a single organism (“superorganism”) and its elements as “tissues” or, arguably, even “organs”. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

The Life Cycle of Trypanosoma Cruzi
Tyler et al. THE LIFE CYCLE OF TRYPANOSOMA CRUZI K. M. Tyler, C. L. Olson and D. M. Engman Departments of Microbiology-Immunology and Pathology Feinberg Medical School of Northwestern University, Chicago, IL 60611 ABSTRACT Since the discovery of Trypanosoma cruzi as the parasite that causes Chagas disease, nearly a century ago, the details of the organism's life cycle have fascinated scientists. T. cruzi is a single-celled eukaryote with a complex life cycle alternating between reduviid bug vectors and vertebrate hosts. It is able to adapt via the process of cellular differentiation to replicate within the diverse environments represented of the insect's gut and host cell cytoplasm. These adaptive transformations take the form of coordinated changes in morphology, metabolism and cell cycle regulation. Different life cycle stages of T. cruzi show dramatically different protein and RNA profiles, which are the end result of unusual mechanisms for regulating gene expression. In recent years, new molecular techniques have been brought to bear on the life cycle dramatically increasing our knowledge of the strategies employed by the parasite to ensure its continued survival. INTRODUCTION Chagas disease The etiologic agent of the chronic and often fatal Chagas disease is the American trypanosome, Trypanosoma cruzi , a flagellated protozoan of the order Kinetoplastida. The survival of T. cruzi is dependent on the successful transmission between, and the colonization of, two radically different environments: the midgut of the reduviid bug vector and the cytoplasm of the mammalian host cell. As is true of all infections, interruption of the pathogen's life cycle will lead to eradication of the disease. -

A PRELIMINARY NOTE on TETRAMITUS, a STAGE in the LIFE CYCLE of a COPROZOIC AMOEBA by MARTHA BUNTING
294 Z6OL6G Y.- M. BUNTING PROC. N. A. S. A PRELIMINARY NOTE ON TETRAMITUS, A STAGE IN THE LIFE CYCLE OF A COPROZOIC AMOEBA By MARTHA BUNTING DEPARTMENT OF ZOOLOGY, UNIVERSITY OF PENNSYLVANIA Communicated July 24, 1922 In 1920 a study of the Entamoeba of the rat was begun and in a culture on artificial medium a number of Coprozoic amoeba appeared, one of which exhibited a flagellate phase which seems to be identical with Tetramitus rostratus Perty.1 The appearance of flagellate phases in cultures of Coprozoic amoeba (Whitmore,2 etc.) as well as of soil amoeba (Kofoid,1 Wilson,4 etc.) has been recorded a number of times, but the flagellates which appeared were relatively simple in organization and very transitory in occurrence. Fur- thermore, some of the simpler flagellates have been observed (Pascher5) to lose their flagella and become amoeboid. In the case here described, however, there is a transformation of a simple amoeba into a relatively complex flagellate which multiplies for several days then returns to the amoeboid condition and becomes encysted. Tetramitus rostratus has been studied by a number of investigators since its discovery in 1852, by Perty,' yet no one has given a full account of its life history. The lack of cysts in previous accounts, mentioned by Dobell and O'Connor,6 is explained by the transformation into an amoeba before encystment. It is believed that this animal presents the extreme in transformations of this sort and serves to emphasize the close relationship between amoebae and flagellates, and the need for careful studies of life cycles, in pure cultures where possible, in investigations of coprozoic and other Protozoa.