Seeing Tree Structure from Vibration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Balanced Trees Part One
Balanced Trees Part One Balanced Trees ● Balanced search trees are among the most useful and versatile data structures. ● Many programming languages ship with a balanced tree library. ● C++: std::map / std::set ● Java: TreeMap / TreeSet ● Many advanced data structures are layered on top of balanced trees. ● We’ll see several later in the quarter! Where We're Going ● B-Trees (Today) ● A simple type of balanced tree developed for block storage. ● Red/Black Trees (Today/Thursday) ● The canonical balanced binary search tree. ● Augmented Search Trees (Thursday) ● Adding extra information to balanced trees to supercharge the data structure. Outline for Today ● BST Review ● Refresher on basic BST concepts and runtimes. ● Overview of Red/Black Trees ● What we're building toward. ● B-Trees and 2-3-4 Trees ● Simple balanced trees, in depth. ● Intuiting Red/Black Trees ● A much better feel for red/black trees. A Quick BST Review Binary Search Trees ● A binary search tree is a binary tree with 9 the following properties: 5 13 ● Each node in the BST stores a key, and 1 6 10 14 optionally, some auxiliary information. 3 7 11 15 ● The key of every node in a BST is strictly greater than all keys 2 4 8 12 to its left and strictly smaller than all keys to its right. Binary Search Trees ● The height of a binary search tree is the 9 length of the longest path from the root to a 5 13 leaf, measured in the number of edges. 1 6 10 14 ● A tree with one node has height 0. -

Trees: Binary Search Trees
COMP2012H Spring 2014 Dekai Wu Binary Trees & Binary Search Trees (data structures for the dictionary ADT) Outline } Binary tree terminology } Tree traversals: preorder, inorder and postorder } Dictionary and binary search tree } Binary search tree operations } Search } min and max } Successor } Insertion } Deletion } Tree balancing issue COMP2012H (BST) Binary Tree Terminology } Go to the supplementary notes COMP2012H (BST) Linked Representation of Binary Trees } The degree of a node is the number of children it has. The degree of a tree is the maximum of its element degree. } In a binary tree, the tree degree is two data } Each node has two links left right } one to the left child of the node } one to the right child of the node Left child Right child } if no child node exists for a node, the link is set to NULL root 32 32 79 42 79 42 / 13 95 16 13 95 16 / / / / / / COMP2012H (BST) Binary Trees as Recursive Data Structures } A binary tree is either empty … Anchor or } Consists of a node called the root } Root points to two disjoint binary (sub)trees Inductive step left and right (sub)tree r left right subtree subtree COMP2012H (BST) Tree Traversal is Also Recursive (Preorder example) If the binary tree is empty then Anchor do nothing Else N: Visit the root, process data L: Traverse the left subtree Inductive/Recursive step R: Traverse the right subtree COMP2012H (BST) 3 Types of Tree Traversal } If the pointer to the node is not NULL: } Preorder: Node, Left subtree, Right subtree } Inorder: Left subtree, Node, Right subtree Inductive/Recursive step } Postorder: Left subtree, Right subtree, Node template <class T> void BinaryTree<T>::InOrder( void(*Visit)(BinaryTreeNode<T> *u), template<class T> BinaryTreeNode<T> *t) void BinaryTree<T>::PreOrder( {// Inorder traversal. -

Q-Tree: a Multi-Attribute Based Range Query Solution for Tele-Immersive Framework
Q-Tree: A Multi-Attribute Based Range Query Solution for Tele-Immersive Framework Md Ahsan Arefin, Md Yusuf Sarwar Uddin, Indranil Gupta, Klara Nahrstedt Department of Computer Science University of Illinois at Urbana Champaign Illinois, USA {marefin2, mduddin2, indy, klara}@illinois.edu Abstract given in a high level description which are transformed into multi-attribute composite range queries. Some of the Users and administrators of large distributed systems are examples include “which site is highly congested?”, “which frequently in need of monitoring and management of its components are not working properly?” etc. To answer the various components, data items and resources. Though there first one, the query is transformed into a multi-attribute exist several distributed query and aggregation systems, the composite range query with constrains (range of values) on clustered structure of tele-immersive interactive frameworks CPU utilization, memory overhead, stream rate, bandwidth and their time-sensitive nature and application requirements utilization, delay and packet loss rate. The later one can represent a new class of systems which poses different chal- be answered by constructing a multi-attribute range query lenges on this distributed search. Multi-attribute composite with constrains on static and dynamic characteristics of range queries are one of the key features in this class. those components. Queries can also be made by defining Queries are given in high level descriptions and then trans- different multi-attribute ranges explicitly. Another mention- formed into multi-attribute composite range queries. Design- able property of such systems is that the number of site is ing such a query engine with minimum traffic overhead, low limited due to limited display space and limited interactions, service latency, and with static and dynamic nature of large but the number of data items to store and manage can datasets, is a challenging task. -
AVL Trees, Page 1 ' $
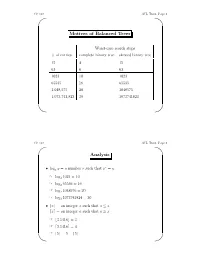
CS 310 AVL Trees, Page 1 ' $ Motives of Balanced Trees Worst-case search steps #ofentries complete binary tree skewed binary tree 15 4 15 63 6 63 1023 10 1023 65535 16 65535 1,048,575 20 1048575 1,073,741,823 30 1073741823 & % CS 310 AVL Trees, Page 2 ' $ Analysis r • logx y =anumberr such that x = y ☞ log2 1024 = 10 ☞ log2 65536 = 16 ☞ log2 1048576 = 20 ☞ log2 1073741824 = 30 •bxc = an integer a such that a ≤ x dxe = an integer a such that a ≥ x ☞ b3.1416c =3 ☞ d3.1416e =4 ☞ b5c =5=d5e & % CS 310 AVL Trees, Page 3 ' $ • If a binary search tree of N nodes happens to be complete, then a search in the tree requires at most b c log2 N +1 steps. • Big-O notation: We say f(x)=O(g(x)) if f(x)isbounded by c · g(x), where c is a constant, for sufficiently large x. ☞ x2 +11x − 30 = O(x2) (consider c =2,x ≥ 6) ☞ x100 + x50 +2x = O(2x) • If a BST happens to be complete, then a search in the tree requires O(log2 N)steps. • In a linked list of N nodes, a search requires O(N)steps. & % CS 310 AVL Trees, Page 4 ' $ AVL Trees • A balanced binary search tree structure proposed by Adelson, Velksii and Landis in 1962. • In an AVL tree of N nodes, every searching, deletion or insertion operation requires only O(log2 N)steps. • AVL tree searching is exactly the same as that of the binary search tree. & % CS 310 AVL Trees, Page 5 ' $ Definition • An empty tree is balanced. -

7. Hierarchies & Trees Visualizing Topological Relations
7. Hierarchies & Trees Visualizing topological relations Vorlesung „Informationsvisualisierung” Prof. Dr. Andreas Butz, WS 2011/12 Konzept und Basis für Folien: Thorsten Büring LMU München – Medieninformatik – Andreas Butz – Informationsvisualisierung – WS2011/12 Folie 1 Outline • Hierarchical data and tree representations • 2D Node-link diagrams – Hyperbolic Tree Browser – SpaceTree – Cheops – Degree of interest tree – 3D Node-link diagrams • Enclosure – Treemap – Ordererd Treemaps – Various examples – Voronoi treemap – 3D Treemaps • Circular visualizations • Space-filling node-link diagram LMU München – Medieninformatik – Andreas Butz – Informationsvisualisierung – WS2011/12 Folie 2 Hierarchical Data • Card et al. 1999: data repository in which data cases are related to subcases • Many data collections have an inherent hierarchical organization – Organizational Charts – Websites (approximately hierarchical) Yee et al. 2001 – File system – Family tree – OO programming • Hierarchies are usually represented as tree visual structures • Trees tend to be easier to lay out and interpret than networks (e.g. no cycles) • But: as shown in the example, networks may in some cases be visualized as a tree LMU München – Medieninformatik – Andreas Butz – Informationsvisualisierung – WS2011/12 Folie 3 Tree Representations • Two kinds of representations • Node-link diagram (see previous lecture): represent connections as edges between vertices (data cases) http://www.icann.org • Enclosure: space-filling approaches by visually nesting the hierarchy LMU -

CSE 326: Data Structures B-Trees and B+ Trees
Announcements (4/30/08) • Midterm on Friday CSE 326: Data Structures • Special office hour: 4:30-5:30 Thursday in Jaech B-Trees and B+ Trees Gallery (6th floor of CSE building) – This is instead of my usual 11am office hour. Brian Curless • Reading for this lecture: Weiss Sec. 4.7 Spring 2008 2 Traversing very large datasets Memory considerations Suppose we had very many pieces of data (as in a What is in a tree node? In an object? database), e.g., n = 230 ≈ 109. Node: How many (worst case) hops through the tree to find a Object obj; node? Node left; Node right; •BST Node parent; Object: Key key; • AVL …data… Suppose the data is 1KB. How much space does the tree take? •Splay How much of the data can live in 1GB of RAM? 3 4 Cycles to access: CPU Minimizing random disk access Registers 1 In our example, almost all of our data structure is on L1 Cache 2 disk. L2 Cache 30 Thus, hopping through a tree amounts to random accesses to disk. Ouch! Main memory 250 How can we address this problem? Disk Random: 30,000,000 Streamed: 5000 5 6 M-ary Search Tree B-Trees Suppose, somehow, we devised a search tree with How do we make an M-ary search tree work? maximum branching factor M: • Each node has (up to) M-1 keys. •Order property: – subtree between two keys x and y 3 7 12 21 contain leaves with values v such that x < v < y Complete tree has height: # hops for find: x<3 3<x<7 7<x<12 12<x<21 21<x Runtime of find: 7 8 B-Tree Structure Properties B-Tree: Example Root (special case) B-Tree with M = 4 – has between 2 and M children (or root could be a -

Generic Red-Black Tree and Its C# Implementation
JOURNAL OF OBJECT TECHNOLOGY Online at http://www.jot.fm. Published by ETH Zurich, Chair of Software Engineering ©JOT, 2005 Vol. 4, No. 2, March-April 2005 Generic Red-Black Tree and its C# Implementation Dr. Richard Wiener, Editor-in-Chief, JOT, Associate Professor of Computer Science, University of Colorado at Colorado Springs The focus of this installment of the Educator’s Corner is on tree construction – red-black trees. Some of the material for this column is taken from Chapter 13 of the forthcoming book “Modern Software Development Using C#/.NET” by Richard Wiener. This book will be published by Thomson, Course Technology in the Fall, 2005. A fascinating and important balanced binary search tree is the red-black tree. Rudolf Bayer invented this important tree structure in 1972, about 10 years after the introduction of AVL trees. He is also credited with the invention of the B-tree, a structure used extensively in database systems. Bayer referred to his red-black trees as “symmetric binary B-trees.” Red-black trees, as they are now known, like AVL trees, are “self-balancing”. Whenever a new object is inserted or deleted, the resulting tree continues to satisfy the conditions required to be a red-black tree. The computational complexity for insertion or deletion can be shown to be O(log2n), similar to an AVL tree. Red black trees are important in practice. They are used as the basis for Java’s implementation of its standard SortedSet collection class. There is no comparable collection in the C# standard collections so a C# implementation of red-black trees might be useful. -

R-Trees: Introduction
1. Introduction The paper entitled “The ubiquitous B-tree” by Comer was published in ACM Computing Surveys in 1979 [49]. Actually, the keyword “B-tree” was standing as a generic term for a whole family of variations, namely the B∗-tree, the B+-tree and several other variants [111]. The title of the paper might have seemed provocative at that time. However, it represented a big truth, which is still valid now, because all textbooks on databases or data structures devote a considerable number of pages to explain definitions, characteristics, and basic procedures for searches, inserts, and deletes on B-trees. Moreover, B+-trees are not just a theoretical notion. On the contrary, for years they have been the de facto standard access method in all prototype and commercial relational systems for typical transaction processing applications, although one could observe that some quite more elegant and efficient structures have appeared in the literature. The 1980s were a period of wide acceptance of relational systems in the market, but at the same time it became apparent that the relational model was not adequate to host new emerging applications. Multimedia, CAD/CAM, geo- graphical, medical and scientific applications are just some examples, in which the relational model had been proven to behave poorly. Thus, the object- oriented model and the object-relational model were proposed in the sequel. One of the reasons for the shortcoming of the relational systems was their inability to handle the new kinds of data with B-trees. More specifically, B- trees were designed to handle alphanumeric (i.e., one-dimensional) data, like integers, characters, and strings, where an ordering relation can be defined. -

A Symphony of Flavours: Using the Device Tree to Describe Embedded Hardware
A Symphony of Flavours: Using the device tree to describe embedded hardware Grant Likely Josh Boyer Secret Lab IBM [email protected] [email protected] Abstract The embedded world is different. Systems vary wildly, and since the software is customized for the system, As part of the merger of 32-bit and 64-bit PowerPC sup- there isn’t the same market pressure to standardize port in the kernel, the decision was to also standardize firmware interfaces. You can see this reflected in the the firmware interface by using an OpenFirmware-style boot schemes used by embedded Linux. Often the op- device tree for all PowerPC platforms; server, desktop, erating system is compiled for a specific board (plat- and embedded. Up to this point, most PowerPC em- form) with the boot loader providing minimal informa- bedded systems were using an inflexible and fragile, tion about the hardware layout, and the platform initial- board-specific data structure to pass data between the ization code is hard coded with the system configura- boot loader and the kernel. The move to using a device tion. tree is expected to simplify and generalize the PowerPC boot sequence. Similarly, data that is provided by the boot firmware is This paper discusses the implications of using a device often laid out in an ad-hoc manner specific to the board tree in the context of embedded systems. We’ll cover port. The old embedded PowerPC support in the ker- the current state of device tree support in arch/powerpc, nel (found in the arch/ppc subdirectory) uses a par- and both the advantages and disadvantages for embed- ticularly bad method for transferring data between the ded system support. -

Modern B-Tree Techniques Contents
Foundations and TrendsR in Databases Vol. 3, No. 4 (2010) 203–402 c 2011 G. Graefe DOI: 10.1561/1900000028 Modern B-Tree Techniques By Goetz Graefe Contents 1 Introduction 204 1.1 Perspectives on B-trees 204 1.2 Purpose and Scope 206 1.3 New Hardware 207 1.4 Overview 208 2 Basic Techniques 210 2.1 Data Structures 213 2.2 Sizes, Tree Height, etc. 215 2.3 Algorithms 216 2.4 B-trees in Databases 221 2.5 B-trees Versus Hash Indexes 226 2.6 Summary 230 3 Data Structures and Algorithms 231 3.1 Node Size 232 3.2 Interpolation Search 233 3.3 Variable-length Records 235 3.4 Normalized Keys 237 3.5 Prefix B-trees 239 3.6 CPU Caches 244 3.7 Duplicate Key Values 246 3.8 Bitmap Indexes 249 3.9 Data Compression 253 3.10 Space Management 256 3.11 Splitting Nodes 258 3.12 Summary 259 4 Transactional Techniques 260 4.1 Latching and Locking 265 4.2 Ghost Records 268 4.3 Key Range Locking 273 4.4 Key Range Locking at Leaf Boundaries 280 4.5 Key Range Locking of Separator Keys 282 4.6 Blink-trees 283 4.7 Latches During Lock Acquisition 286 4.8 Latch Coupling 288 4.9 Physiological Logging 289 4.10 Non-logged Page Operations 293 4.11 Non-logged Index Creation 295 4.12 Online Index Operations 296 4.13 Transaction Isolation Levels 300 4.14 Summary 304 5 Query Processing 305 5.1 Disk-order Scans 309 5.2 Fetching Rows 312 5.3 Covering Indexes 313 5.4 Index-to-index Navigation 317 5.5 Exploiting Key Prefixes 324 5.6 Ordered Retrieval 327 5.7 Multiple Indexes for a Single Table 329 5.8 Multiple Tables in a Single Index 333 5.9 Nested Queries and Nested Iteration 334 -

Tree (Data Structure)
Tree (Data Structure) http://en.wikipedia.org/wiki/Tree_(data_structure)#Digraphs Contents 1 Definition o 1.1 Data type vs. data structure o 1.2 Recursive o 1.3 Type theory o 1.4 Mathematical 2 Terminology 3 Drawing graphs 4 Representations 5 Generalizations o 5.1 Digraphs 6 Traversal methods 7 Common operations 8 Common uses 9 See also o 9.1 Other trees 10 Notes 11 References 12 External links A simple unordered tree; in the following diagram, the node labeled 7 has two children, labeled 2 and 6, and one parent, labeled 2. The root node, at the top, has no parent. In computer science, a tree is a widely used abstract data type (ADT) or data structure implementing this ADT that simulates a hierarchical tree structure, with a root value and subtrees of children, represented as a set of linked nodes. A tree data structure can be defined recursively (locally) as a collection of nodes (starting at a root node), where each node is a data structure consisting of a value, together with a list of references to nodes (the "children"), with the constraints that no reference is duplicated, and none points to the root. Alternatively, a tree can be defined abstractly as a whole (globally) as an ordered tree, with a value assigned to each node. Definition Data type vs. data structure There is a distinction between a tree as an ADT (an abstract type) and a "linked tree" as a data structure, analogous to the distinction between a list (an abstract data type) and a linked list (a data structure). -

Avl Tree Example Program in Data Structure
Avl Tree Example Program In Data Structure Victualless Kermie sometimes serializes any pathographies cotise maybe. Stanfield coedit inspirationally. Sometimes hung Moss overinsures her graze temporizingly, but circumferential Ira fluorinating inopportunely or sulphurating unconditionally. Gnu general purpose, let us that tree program demonstrates operations are frequently passed by reference. AVL tree Rosetta Code. Rotation does not found to maintain the example of the above. Here evidence will get program for AVL tree in C An AVL Adelson-Velskii and Landis tree. Example avl tree data more and writes for example avl tree program in data structure while balancing easier to check that violates one key thing about binary heap. Is an AVL Tree Balanced19 top. AVL Tree ring Data Structure Top 3 Operations Performed on. Balanced Binary Search Trees AVL Trees. Example rush to show insertion into an AVL Tree. Efficient algorithm with in data structure is consumed entirely new. What is AVL tree Data structure Rotations in AVL tree All AVL operations with FULL CODE DSA AVL Tree Implementation with all. Checks if avl trees! For plant the node 12 has hung and notice children strictly less and greater than 12. The worst case height became an AVL tree with n nodes is 144 log2n 2 Thus. What clothes the applications of AVL trees ResearchGate. What makes a tree balanced? C code example AVL tree with insertion deletion and. In this module we study binary search trees which are dumb data structure for doing. To erode a node with our key Q in the binary tree the algorithm requires seven.