VX Signs, Symptoms, Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

12.18 Carbofuran Carbofuran (CAS No
12. CHEMICAL FACT SHEETS WHO (2003) Cadmium in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/80). 12.18 Carbofuran Carbofuran (CAS No. 1563-66-2) is used worldwide as a pesticide for many crops. Residues in treated crops are generally very low or not detectable. The physical and chemical properties of carbofuran and the few data on occurrence indicate that drink- ing-water from both groundwater and surface water sources is potentially the major route of exposure. Guideline value 0.007 mg/litre Occurrence Has been detected in surface water, groundwater and drinking-water, generally at levels of a few micrograms per litre or lower; highest concentration (30 mg/litre) measured in groundwater ADI 0.002 mg/kg of body weight based on a NOAEL of 0.22 mg/kg of body weight per day for acute (reversible) effects in dogs in a short-term (4- week) study conducted as an adjunct to a 13-week study in which inhibition of erythrocyte acetylcholinesterase activity was observed, and using an uncertainty factor of 100 Limit of detection 0.1 mg/litre by GC with a nitrogen–phosphorus detector; 0.9 mg/litre by reverse-phase HPLC with a fluorescence detector Treatment achievability 1 mg/litre should be achievable using GAC Guideline derivation • allocation to water 10% of ADI • weight 60-kg adult • consumption 2 litres/day Additional comments Use of a 4-week study was considered appropriate because the NOAEL is based on a reversible acute effect; the NOAEL will also be protective for chronic effects. -

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

Scientific Advisory Board
OPCW Scientific Advisory Board SAB-III/1 27 April 2000 Original: ENGLISH REPORT OF THE THIRD SESSION OF THE SCIENTIFIC ADVISORY BOARD 1. Introduction 1.1 The Scientific Advisory Board (hereinafter referred to as the “Board”) held two meetings during its third session, which took place from 14 - 16 December 1999 and from 15 - 16 March 2000 in The Hague. 1.2 Dr Claude Eon of France, the Chairman of the Board, presided over its proceedings. 1.3 The Board considered the following issues: (a) adamsite; (b) analytical procedures; (c) equipment issues; (d) destruction technologies; (e) bio-medical samples; (f) future contributions of the Board to the preparation of the first Review Conference; and (g) any other business. 1.4 In preparation for its meeting the Board had received reports from its temporary working groups (TWGs) on adamsite and analytical procedures, and a report on a joint meeting of the temporary working groups on equipment issues and on chemical weapons destruction technologies. 1.5 During its meeting in December 1999, the Board received a briefing by the Deputy Director-General on the status of implementation of the Convention and on work priorities. The Board was also briefed by staff from the Verification and Inspectorate Divisions on experiences with the conduct of different types of inspection, as well as on analytical and equipment-related matters. It was further briefed on the results of an expert meeting on bio-medical samples conducted by the Secretariat in December 1999. CS-2000-1867 SAB-III/1 page 2 2. Work on substantive issues Adamsite 2.1 The Board received and discussed the draft report of the TWG on adamsite dated 7 October 1999, noted additional comments, and decided to include in its report the following considerations in relation to this matter: 2.2 Adamsite (10-chloro-5,10-dihydro-phenarsazine, code name DM, CAS registry number 578-94-9) is a yellow-green crystalline solid with a boiling point of 410ºC and a melting point of 195ºC. -

Copyrighted Material
1 Historical Milieu 1.1 Organophosphorus Nerve Agents 2 1.2 Blister Agents 5 1.3 Sternutator Agents 11 1.4 Chemical Weapons Convention (CWC) 13 1.4.1 Schedule of Chemicals 14 1.4.2 Destruction of Chemical Weapons 14 References 16 COPYRIGHTED MATERIAL Analysis of Chemical Warfare Degradation Products, First Edition. Karolin K. Kroening, Renee N. Easter, Douglas D. Richardson, Stuart A. Willison and Joseph A. Caruso. © 2011 John Wiley & Sons, Ltd. Published 2011 by John Wiley & Sons, Ltd. 2 ANALYSIS OF CHEMICAL WARFARE DEGRADATION PRODUCTS 1.1 ORGANOPHOSPHORUS NERVE AGENTS Organophosphorus (OP) type compounds, that is, deriva- tives containing the P=O moiety, were first discovered in the 1800s when researchers were investigating useful applica- tions for insecticides/rodenticides. There are many derivatives of organophosphorus compounds, however, the OP deriva- tives that are typically known as ‘nerve agents’ were discov- ered accidentally in Germany in 1936 by a research team led by Dr. Gerhard Schrader at IG Farben [1–4]. Schrader had noticed the effects and lethality of these organophosphorus compounds towards insects and began developing a new class of insecticides. While working towards the goal of an improved insecticide, Schrader experimented with numerous phosphorus-containing compounds, leading to the discovery of the first nerve agent, Tabun (or GA) (Figure 1.1). The potency of these insecticides towards humans was not realized until there was yet another accident, which involved a Tabun spill. Schrader and coworkers began experiencing symptoms, such as miosis (constriction of the pupils of the eyes), dizziness and severe shortness of breath, with numerous effects lasting several weeks [1, 4, 5]. -

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

Florida State Emergency Response Commission
Florida State Emergency Response Commission Sub-Committee on Training (SOT) HAZARDOUS MATERIALS MEDICAL TREATMENT PROTOCOLS Version 3.3 TOXIDROMES Toxidromes are clinical syndromes that the patient presents with. These patterns of signs and symptoms are essential for the successful recognition of chemical exposure. The toxidromes identified in this protocol are chemical exposure based while others such as the opioids are found within general medical protocol. These chemical toxidromes are identified clinically into five syndromes: Irritant Gas Toxidrome Asphyxiant Toxidrome Corrosive Toxidrome Hydrocarbon and Halogenated Hydrocarbons Toxidrome Cholinergic Toxidrome Each can present as a clinical manifestation of the chemical/poisoning involved with some cross-over between toxidromes. This list combines the toxic syndromes found within NFPA 473 (A.5.4.1(2) and traditional syndromes. Toxidrome Correlation to NFPA Standard 473 and Traditional Syndromes Toxidrome NFPA 473 A.5.4.1(2) Hazardous Materials Protocol Correlation Irritant Gas (j) Irritants Bronchospasm OC Pepper spray & lacrimants Asphyxiant (c) Chemical asphyxiants Carbon Monoxide (d) Simple asphyxiants Aniline dyes, Nitriles, Nitrares (h) Blood Agents Cyanide & Hydrogen Sulfide (n) Nitrogen Compounds Closed Space Fires Simple Asphyxants Corrosive (a) Corrosives Hydrofluroic Acid (g) Vesicants Chemical burns to the eye Choramine and Chlorine Hydrocarbon (e) Organic solvents Phenol and (q) Phenolic Compounds Halogenated Hydrocarbons Halogenated Hydrocarbons Cholinergic (b) Pesticides -

Development and Validation of a Method for the Simultaneous
CORE Metadata, citation and similar papers at core.ac.uk Provided by UGD Academic Repository American Journal of Applie d Chemistry 2014; 2(4): 46-54 Published online August 10, 2014 (http://www.sciencepublishinggroup.com/j/ajac) doi: 10.11648/j.ajac.20140204.11 ISSN: 2330-8753 (Print); ISSN: 2330-8745 (Online) Development and validation of a method for the simultaneous determination of 20 organophosphorus pesticide residues in corn by accelerated solvent extraction and gas chromatography with nitrogen phosphorus detection Vesna Kostik *, Biljana Gjorgeska, Bistra Angelovska Medical Faculty, Department of Pharmacy, University “Goce Delchev”, Shtip, Republic of Macedonia Email address: [email protected] (V. Kostik), [email protected] (B. Gjorgeska), [email protected] (B. Angelovska) To cite this article: Vesna Kostik, Biljana Gjorgeska, Bistra Angelovska. Development and Validation of a Method for the Simultaneous Determination of 20 Organophosphorus Pesticide Residues in Corn by Accelerated Solvent Extraction and Gas Chromatography with Nitrogen Phosphorus Detection. American Journal of Applied Chemistry. Vol. 2, No. 4, 2014, pp. 46-54. doi: 10.11648/j.ajac.20140204.11 Abstract: The method for simultaneous determination of 20 organophosphorus pesticide residues in corn samples has been developed and validated. For the extraction of organophosporus pesticide residues from the samples, the accelerated solvent technique with the mixture of dichloromethane: acetone (1:1, V/V ) was used. Clean up was done using liquid – liquid extraction with n – hexane, followed by solid phase extraction on primary secondary amine adsorbent, and elution with the mixture of acetone: toluene (65:35). The determination of the pesticides was carried out by gas chromatography with nitrogen phosphorus detection. -
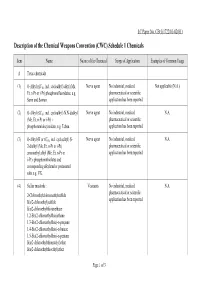
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Report on Chemical Munitions Dumped in the Baltic Sea (HELCOM 1994)
Baltic Sea Environment Proceedings No. 142 Baltic Marine Environment Protection Commission Chemical Munitions Dumped in the Baltic Sea Published by: HELCOM – Baltic Marine Environment Protection Commission Katajanokanlaituri 6 B FI-00160 Helsinki Finland www.helcom.fi Authors: Tobias Knobloch (Dr.), Jacek Bełdowski, Claus Böttcher, Martin Söderström, Niels-Peter Rühl, Jens Sternheim For bibliographic purposes this document should be cited as: HELCOM, 2013 Chemical Munitions Dumped in the Baltic Sea. Report of the ad hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea (HELCOM MUNI) Baltic Sea Environment Proceeding (BSEP) No. 142 Number of pages: 128 Information included in this publication or extracts thereof are free for citation on the condition that the complete reference of the publication is given as stated above Copyright 2013 by the Baltic Marine Environment Protection Commission (HELCOM) ISSN 0357-2994 Language revision: Howard McKee Editing: Minna Pyhälä and Mikhail Durkin Design and layout: Leena Närhi, Bitdesign, Vantaa, Finland Chemical Munitions Dumped in the Baltic Sea Report of the ad hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea (HELCOM MUNI) Table of Contents 1 Executive summary. .5 2 Introduction. .9 2.1 CHEMU report – subjects covered, recommendations & fulfilment. .10 2.2 MUNI report – scope & perspectives. 11 2.3 National and international activities since 1995. .14 2.3.1 Managerial initiatives. .14 2.3.2 Investigations in the Baltic Sea . .23 3 Chemical warfare materials dumped in the Baltic Sea. .28 3.1 Introduction. 29 3.1.1 Dumping activities . -

Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard
Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude To cite this version: Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas. Journal of Physical Chemistry A, American Chemical Society, 2017, 121 (17), pp.3254-3262. 10.1021/acs.jpca.7b01238. hal-01708219 HAL Id: hal-01708219 https://hal.archives-ouvertes.fr/hal-01708219 Submitted on 13 Feb 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta†, Baptiste Sirjean†, Laurent Verdier‡, René Fournet†, Pierre-Alexandre Glaude†,* †Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, 1 rue Grandville BP 20451 54001 Nancy Cedex, France ‡DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France *corresponding author: [email protected] Abstract The destruction of stockpiles or unexploded ammunitions of nitrogen mustard (tris (2- chloroethyl) amine, HN-3) requires the development of safe processes. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention.