Development and Validation of a Method for the Simultaneous
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Processing on 14 C-Chlofenvinphos Residues In
EG0800342 The Effect of Processing on 14C- Chlorfenvinphos Residues in Maize Oil and Bioavailability of its Cake Residues on Rats F. Mahdy, S. El-Maghraby National Research Centre, Dokki, Giza, Egypt ABSTRACT Maize seed obtained from 14C-chlorfenvinphos treated plants contained 0.12 % of the applied dose. The insecticide residues in crude oil, methanol and coke amounted to 10 %, 6 % and 69 %, respectively of original residues inside the seeds.The 14C-activity in the crude oil could be a gradual reduced by the refining processes. The alkali treatment and bleaching steps are more effective steps in the refining processes remove about (63 %). The refined oil contained only about 17 % of the 14C-residues originally present. The major residues in processed oil contain parent compound, in addition to five metabolites of the insecticide. When rats fed the extracted seeds (cake), the bound residues were found to be considerably bioavailable. After feeding rats for 5 days with the cake, a substantial amount of 14C-residues was eliminated in the urine (59.5 %), while about 20 % was excreted in the feces. About 15 % of the radioactivity was distribution among various organs. Keywords: 14C-chlorfenvinphos, maize oil, refining processes, residues, bioavailability. INTRODUCTION Pesticides are of interest as residues because of their widespread use in a variety of crops for field and post-harvest protection and because with their degradation products, they could be a potential health hazard. Past investigations suggest that most organochlorine and organophosphorus pesticide in edible oils can be reduced considerably by a chemical refining process, but pyrethroid pesticides remain to a certain extent (1-6). -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2017 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2017 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Note: Chemicals may be added to or deleted from the list. The Emergency Planning and Community Right-to-Know Call Center or the TRI-Listed Chemicals website will provide up-to-date information on the status of these changes. See section B.3.c of the instructions for more information on the de minimis % limits listed below. There are no de minimis levels for PBT chemicals since the de minimis exemption is not available for these chemicals (an asterisk appears where a de minimis limit would otherwise appear in Table II). However, for purposes of the supplier notification requirement only, such limits are provided in Appendix C. Chemical Qualifiers Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. An EPCRA Section 313 chemical that is listed without a qualifier is subject to reporting in all forms in which it is manufactured, processed, and otherwise used. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical/ Chemical Category CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. -

Analysis of Organophosphorus Pesticides in Drinking Water Using
Prepared by: T. Dobbs, J. Netzer, J. Salmons and J. Wiseman J2 Scientific, 1901 Pennsylvania Drive, Suite C, Columbia, MO 65202 Contact Information: [email protected]; 573-214-0472 Why Should We Measure? Organophosphorus compounds are a very major class of pesticides and are exported to almost every country in the world, well over 77 million pounds/year in US alone. Most intended uses are for row crops, but are also used for mosquito control as well as household and garden pests thereby increasing exposure risks. Contamination from these compounds in water must be monitored due to their acetylcholinesterase deactivation potential. Monitoring data exists for most pesticides including OP’s in drinking water sources. Very little monitoring data until recently of OP pestidices under drinking water conditions Some OP’s are partially removed by DW treatment proceses, but others may be transformed into contaminants which are equally or more toxic than the parent compound. Method Considerations Large numbers of samples require a method which can be run unattended. Requires minimum sample prep Minimum of sample manipulations Steps to be Automated: 1 Liter samples requiring no pre-extraction Large Volume sample through the cartridge in a minimum amount of time Drying of Cartridge with no additional manipulation Automatic elution and concentration to GC vial All surfaces appropriately rinsed to prevent carry-over An Automated Solution PrepLinc SPEi System with AccuVap FLX • Automated, programmable introduction of sample to SPE -

Amending Annex II to Directives 76/895/EEC and 86/362/EEC
20 . 5 . 88 Official Journal of the European Communities No L 126/53 COUNCIL DIRECTIVE of 16 May 1988 amending Annex II to Directives 76/895/EEC and 86/362/EEC relating to the fixing of maximum levels for pesticide residues in and on fruit and vegetables and cereals respectively (88/298/EEC) THE COUNCIL OF THE EUROPEAN COMMUNITIES, Having regard to the Treaty establishing the European Economic Community, Having regard to Council Directive 76/895/EEC of 23 November 1976 relating to the fixing of maximum levels for pesticide residues in and on fruit and vegetables 0), as last amended by Regulation (EEC) No 3768/85 (2), and in particular Article 5 thereof, Having regard to Council Directive 86/362/EEC of 24 July 1986 on the fixing of maximum levels for pesticide residues in and on cereals (3), and in particular Article 1 1 thereof, Having regard to the proposal from the Commission, Whereas, in the light of technical and scientific progress and of the requirements of public health and agriculture, it is necessary to amend the provisions, particularly the maximum levels, contained in Annex II to Directive 76/895/EEC relating to captafol, captan, chlorfenvinphos, dodine, fenitrothion, folpet, formothion and malathion ; Whereas, for the same reasons, it seems desirable to update Directive 76/895/EEC by adding provisions relating to further pesticides, residues of which may occur in and on fruit and vegetables, namely, ethion, ethylene dibromide, mevinphos, phosalone and 2, 4, 5-T, arid to update Directive 86/362/EEC by adding provisions relating to a further pesticide the residues of which may occur in cereals, namely, captafol, HAS ADOPTED THIS DIRECTIVE : Article 1 Annex II to Directive 76/895/EEC is hereby amended as follows : 1 . -

SECRETARY HICKEL BANS USE of 16 PESTICIDES on ANY INTERIOR LANDS OR PROGRAMS -- June 18, 1970
i DEPARTMENT of the INTERIOR news release OFFICE OF THE SECRETARY For Release Thursday, June 18, 1970 SECRETARYHICKEL BANS USE OF 16 PESTICIDES ON ANY INTERIOR LANDS OR PROGRAMS Secretary of the Interior Walter J. Hickel today announced a new policy flatly banning the use of 16 types of pesticides on any lands managed by the Department's bureaus and agencies, or in any program run by them. The Department administers approximately 70 percent of all federally owned lands. Included in this list of prohibited pesticides are such widely-known substances as DDT, Aldrin, 2, 4, 5,-T, Dieldrin, Endrin, Heptachlor, Lindane and Toxaphene. Also on the list of unconditionally banned items are Amitrol, arsenical compounds (inorganic), Azodrin, Bidrin, DDD (TDE), mercurial compounds, Strobane, and Thallium Sulfate. The Secretary pointed out that nearly all use of these pesticides has been banned on Interior Lands during recent years. The purpose of the new statement, he said, is to establish a policy for guidance of all Interior personnel. Another group of chemical pesticides, titled the Restricted List, are to be used only when non-chemical techniques have been considered and found inadequate, and when use can be limited to small-scale applications. Secretary Hickel emphasized that the use of any chemical pesticide by his agencies must be aimed at a specific pest problem, and involve minimum strength and minimum frequency of application. "We in the Interior Department-- the Nation's chief conservation agency have a special obligation to protect the environment for all the people,“’ Secretary Hickel said. "We are charged by law with responsibility for protecting interstate and coastal water quality, our fish and wildlife resources, the integrity of our national parks, public lands, and recreation areas," the Secretary continued. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Qsar Analysis of the Chemical Hydrolysis of Organophosphorus Pesticides in Natural Waters
QSAR ANALYSIS OF THE CHEMICAL HYDROLYSIS OF ORGANOPHOSPHORUS PESTICIDES IN NATURAL WATERS. by Kenneth K. Tanji Principal Investigator and Jonathan 1. Sullivan Graduate Research Assistant Department of Land, Air and Water Resources University of California, Davis Technical Completion Report Project Number W-843 August, 1995 University of California Water Resource Center The research leading to this report was supported by the University of California Water Resource Center as part of Water Resource Center Project W-843. Table of Contents Page Abstract 2 Problem and Research Objectives 3 Introduction 5 Theoretical Background 6 QSAR Methodology 7 Molecular Connectivity Theory 8 Organophosphorus Pesticides 12 Experimental Determination of Rates 15 Results and Discussion 17 Principal Findings and Significance 19 References 34 List of Tables Page Table 1. Statistical relationship between OP pesticides and first-order MC/'s. 30 Table 2. Inherent conditions of waters used in experimental work. 16 Table 3. Estimated half-lives for organophosphorus esters derived from model. 31 Table 4. Half-lives and first-order MCI' sfor model calibration data set. 31 Table 5. Experimental kinetic data for validation set compounds, Sacramento. 33 List of Figures Page Figure 1. Essential Features OfQSAR Modeling Methodology. 21 Figure 2. Regression plot for In hydrolysis rate vs. 1st order MCl' s. 22 Figure 3. a 3-D molecular model, a line-segment model and a graphical model. 23 Figure 4. Molecular connectivity index suborders. 24 Figure 5. Chlorpyrifos and its fourteen fourth order path/cluster fragments. 25 Figure 6. Abridged MClndex output. 26 Figure 7. Parent acids of most common organophosphorus pesticides. 12 Figure 8. -

Quantification of Organophosphorus Pesticides by Quechers and LC
HPLC Application ID No.: 20020 Quantification of Organophosphorus Pesticides by QuEchERS and LC/MS/MS Column: Synergi™ 4 µm Fusion-RP 80 Å, LC Column 50 x 2 mm, Ea Dimensions: 50 x 2 mm ID Order No: 00B-4424-B0 Elution Type: Gradient Eluent A: Add 5mL of 1M ammonium formate to 1L Water Eluent B: Add 5mL of 1M ammonium formate to 1L Methanol Gradient Step No. Time (min) Pct A Pct B Profile: 1 -5 95 5 2 0 95 5 3 2 95 5 4 6 50 50 5 12 10 90 6 14 10 90 7 16 95 5 8 17 95 5 Flow Rate: 250 mL/min Col. Temp.: 25 °C Detection: Tandem Mass Spec (MS-MS) @ (ambient) Detector Info: <a target="_blank" href="https://sciex.com/products/mass-spectrometers?utm_campaign=2019%20application%20search&utm_source=phenomenex&utm_medium=referral">SCIEX</a> Analyst Note: SecurityGuard™ Guard Cartridge System extends column lifetime. - SecurityGuard Cartridges, Fusion-RP 4 x 2.0mm ID, 10/Pk Part No.: AJ0-7556 - Holder Part No.: KJ0-4282 ©2021 Phenomenex Inc. All rights reserved. For more information contact your Phenomenex Representative at [email protected] Phenomenex products are available worldwide. www.phenomenex.com [email protected] Page 1 of 2 HPLC Application ID No.: 20020 Quantification of Organophosphorus Pesticides by QuEchERS and LC/MS/MS ANALYTES: 1 Acephate (MRM: 184/142.8) 41 Diazinon (MRM: 305/169) 2 Acephate (MRM: 184/94.9) 42 Diazinon (MRM: 305/153) 3 Demeton-S-methyl sulfone (MRM: 263/169) 43 Fenthion (MRM: 278.9/169) 4 Demeton-S-methyl sulfone (MRM: 263/109) 44 Fenthion (MRM: 278.9/246.9) 5 Parathion-methyl Methyl parathion (MRM: 263.9/232.1)45 -

Pesticides Contamination of Cereals and Legumes: Monitoring of Samples Marketed in Italy As a Contribution to Risk Assessment
applied sciences Article Pesticides Contamination of Cereals and Legumes: Monitoring of Samples Marketed in Italy as a Contribution to Risk Assessment Valeria Nardelli 1, Valeria D’Amico 1, Mariateresa Ingegno 1 , Ines Della Rovere 1, Marco Iammarino 1,* , Francesco Casamassima 1, Anna Calitri 1, Donatella Nardiello 2 , Donghao Li 3 and Maurizio Quinto 2,3,* 1 Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata, Via Manfredonia 20, 71121 Foggia, Italy; [email protected] (V.N.); [email protected] (V.D.); [email protected] (M.I.); [email protected] (I.D.R.); [email protected] (F.C.); [email protected] (A.C.) 2 Dipartimento di Scienze Agrarie, Alimenti, Risorse Naturali e Ingegneria—Università degli Studi di Foggia, Via Napoli, 25, 71122 Foggia, Italy; [email protected] 3 Department of Chemistry, Yanbian University, Park Road 977, Yanji 133002, China; [email protected] * Correspondence: [email protected] (M.I.); [email protected] (M.Q.) Featured Application: This work offers a contribution to risk assessment regarding the levels of 37 pesticides in cereal and legume samples commercialized in Italy during the last years. It is well-known that prolonged exposure to pesticides can increase the risk of cardiovascular and respiratory disease, other than promoting cancer diseases. Thus, the World Health Organization and the European Food Safety Authority ask for monitoring of the levels of such substances, Citation: Nardelli, V.; D’Amico, V.; especially in vegetables, continuously, to have availability updated and detailed data on this Ingegno, M.; Della Rovere, I.; Iammarino, M.; Casamassima, F.; type of food contamination. -
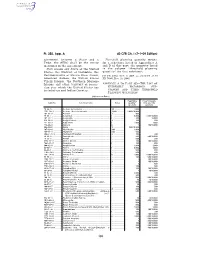
40 CFR Ch. I (7–1–09 Edition) Pt. 355, App. A
Pt. 355, App. A 40 CFR Ch. I (7–1–09 Edition) agreement between a State and a Threshold planning quantity means, Tribe, the SERC shall be the entity for a substance listed in Appendices A identified in the agreement. and B of this part, the quantity listed State means any State of the United in the column ‘‘threshold planning States, the District of Columbia, the quantity’’ for that substance. Commonwealth of Puerto Rico, Guam, [73 FR 65462, Nov. 3, 2008, as amended at 73 American Samoa, the United States FR 76960, Dec. 18, 2008] Virgin Islands, the Northern Mariana Islands, any other territory or posses- APPENDIX A TO PART 355—THE LIST OF sion over which the United States has EXTREMELY HAZARDOUS SUB- jurisdiction and Indian Country. STANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. 10 ................ 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... 1,000 ........... 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... 1 .................. 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f ................... 100 10,000