And Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nomenclature of Carboxylic Acids • Select the Longest Carbon Chain Containing the Carboxyl Group
Chapter 5 Carboxylic Acids and Esters Carboxylic Acids • Carboxylic acids are weak organic acids which Chapter 5 contain the carboxyl group (RCO2H): Carboxylic Acids and Esters O C O H O RCOOH RCO2H Chapter Objectives: O condensed ways of • Learn to recognize the carboxylic acid, ester, and related functional groups. RCOH writing the carboxyl • Learn the IUPAC system for naming carboxylic acids and esters. group a carboxylic acid C H • Learn the important physical properties of the carboxylic acids and esters. • Learn the major chemical reaction of carboxylic acids and esters, and learn how to O predict the products of ester synthesis and hydrolysis reactions. the carboxyl group • Learn some of the important properties of condensation polymers, especially the polyesters. Mr. Kevin A. Boudreaux • The tart flavor of sour-tasting foods is often caused Angelo State University CHEM 2353 Fundamentals of Organic Chemistry by the presence of carboxylic acids. Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea 2 Nomenclature of Carboxylic Acids • Select the longest carbon chain containing the carboxyl group. The -e ending of the parent alkane name is replaced by the suffix -oic acid. • The carboxyl carbon is always numbered “1” but the number is not included in the name. • Name the substituents attached to the chain in the Nomenclature of usual way. • Aromatic carboxylic acids (i.e., with a CO2H Carboxylic Acids directly connected to a benzene ring) are named after the parent compound, benzoic acid. O C OH 3 -

Optimisation Du Métabolisme Énergétique Du Soufre Chez La Bactérie Hyperthermophile Aquifex Aeolicus
Aix-Marseille Université Ecole Doctorale des Sciences de la Vie et de la Santé Thèse de Doctorat d’Université Mention Microbiologie Clément AUSSIGNARGUES Optimisation du métabolisme énergétique du soufre chez la bactérie hyperthermophile Aquifex aeolicus Soutenue le 17 Décembre 2012 devant le jury: Pr. Frédéric BARRAS Dr. Laurent COURNAC Dr. Bruno FRANZETTI Dr. Marie-Thérèse GIUDICI-ORTICONI Dr. Anne GODFROY Dr. Marianne ILBERT Laboratoire de Bioénergétique et Ingénierie des Protéines Centre National de la Recherche Scientifique Aix-Marseille Université Ecole Doctorale des Sciences de la Vie et de la Santé Thèse de Doctorat d’Université Mention Microbiologie Clément AUSSIGNARGUES Optimisation du métabolisme énergétique du soufre chez la bactérie hyperthermophile Aquifex aeolicus Soutenue le 17 Décembre 2012 devant le jury: Pr. Frédéric BARRAS Dr. Laurent COURNAC Dr. Bruno FRANZETTI Dr. Marie-Thérèse GIUDICI-ORTICONI Dr. Anne GODFROY Dr. Marianne ILBERT Laboratoire de Bioénergétique et Ingénierie des Protéines Centre National de la Recherche Scientifique Sommaire Sommaire Introduction 2 Chapitre I- Métabolismes énergétiques du soufre 4 I) Le soufre, un élément essentiel aux multiples facettes 6 II) Le soufre utilisé comme composé énergétique 9 A) Oxydation des composés du soufre 10 1. Oxydation du sulfure d’hydrogène H2S 10 a) La Sulfure Quinone Oxydoréductase (SQR) 10 b) La flavocytochrome c sulfure déshydrogénase (FCSD) 13 2- 2. Oxydation du thiosulfate (S2O3 ) 14 a) Thiosulfate : accepteur oxydoréductase (TAOR) 14 b) Le système Sox 16 2- 3. Oxydation du sulfite (SO3 ) 20 a) Sulfite : accepteur oxydoréductase (SAOR) 21 b) Voie de l’APS 23 4. Oxydation du soufre stocké dans les globules 26 B) La réduction du soufre : soufre/polysulfure réductase 29 C) Un cas particulier : la dismutation du soufre (SOR) 33 1. -

Carboxylic Acid Addition to Terminal Alkynes Utilizing Ammonium Tagged
Journal of Organometallic Chemistry 883 (2019) 11e16 Contents lists available at ScienceDirect Journal of Organometallic Chemistry journal homepage: www.elsevier.com/locate/jorganchem Carboxylic acid addition to terminal alkynes utilizing ammonium tagged Hoveyda-Grubbs catalyst supported on magnetically separable core/shell silica: A highly reusable and air compatible catalytic system € € * ** Bengi Ozgün Oztürk , Didar Gürcü, Solmaz Karabulut S¸ ehitoglu Hacettepe University, Faculty of Science, Chemistry Department, 06800, Beytepe, Ankara, Turkey article info abstract Article history: In this study, the performance of ammonium tagged Hoveyda-Grubbs catalyst supported on magnetically Received 24 October 2018 separable core/shell silica gel was tested on carboxylic acid addition reactions to terminal alkynes using a Received in revised form variety of carboxylic acid derivatives under air atmosphere. The catalytic system was found to be 13 December 2018 compatible with air atmosphere and can tolerate even non-degassed solvents. The reaction parameters Accepted 10 January 2019 such as temperature, substrate/catalyst ratio and the effect of carboxylic acid on the selectivity and yield Available online 14 January 2019 of the reaction were investigated in details. The reaction of arylacetylenes with acetic acid yielded the corresponding E-isomer with conversion values up to 99% with a catalytic loading of 1% Ru. The reus- Keywords: Carboxylic acid ability of the catalyst was tested using acetic acid/benzoic acid and phenylacetylene in toluene at 85 C Alkyne under air atmosphere. The catalyst was found to be highly reusable and maintained its activity up to 11th Ruthenium run, reaching a conversion value of 83% with minimum ruthenium leaching. Reusable catalyst © 2019 Elsevier B.V. -
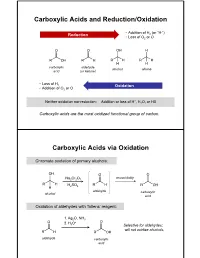
Carboxylic Acids and Reduction/Oxidation Carboxylic
Carboxylic Acids and Reduction/Oxidation • Addition of H (or “H-”) Reduction 2 • Loss of O2 or O carboxylic aldehyde alcohol alkane acid (or ketone) • Loss of H 2 Oxidation • Addition of O2 or O + Neither oxidation nor reduction: Addition or loss of H , H2O, or HX Carboxylic acids are the most oxidized functional group of carbon. Carboxylic Acids via Oxidation Chromate oxidation of primary alcohols: unavoidably Na2Cr2O7 H2SO4 aldehyde carboxylic alcohol acid Oxidation of aldehydes with Tollens’ reagent: 1. Ag2O, NH3 2. H O+ 3 Selective for aldehydes; will not oxidize alcohols. aldehyde carboxylic acid Carboxylic Acids via Oxidation Oxidative cleavage of alkynes: 1. O3 2. H2O internal alkyne Oxidation of benzylic carbons: KMnO4 Purifying Carboxylic Acids by Acid-Base Extraction Acidic and basic organic molecules can be separated from other substances by manipulating their protonation state and solubility. Let’s say we run a reaction. Na2Cr2O7 + unreacted H2SO4 + Na+ 3+ We want this… + Cr(H2O)6 + organic + HSO - …but not these. side products 4 How do we isolate our desired product from the other materials, without using distillation or chromatography? Acid-Base Extraction Step 1: Remove inorganics from organics via differential solubility. a separatory (sept) funnel - Na+ HSO4 Cr(H O) 3+ H2O 2 6 CHCl3 + organic side densities: products (H2O) = 1 g/mL (CHCl3) = 1.48 g/mL A large density difference ensures the two liquids separate completely and quickly. (Much faster than, say, oil and water.) Step 2: Add basic water to deprotonate carboxylic acid, and transfer it to the aqueous phase. - Na+ HSO4 3+ discard Cr(H2O)6 H2O layer + organic + organic side side products products add discard NaOH/ H2O CHCl3 layer + organic side products Step 3: Re-acidify carboxylate to return it to organic solution. -

Chapter 20: Carboxylic Acids and Nitriles
CHAPTER 20 CARBOXYLIC ACIDS Based on McMurry’s Organic Chemistry, 6th edition Dr.Mohanad Al-Hachamii The Importance of Carboxylic Acids (RCO2H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in metabolism Acetic acid, CH3CO2H, - vinegar Butanoic acid, CH3CH2CH2CO2H (rancid butter) Long-chain aliphatic acids from the breakdown of fats Based on McMurry, Organic Chemistry, Chapter 20, 6th edition, (c) 2003 Dr.Mohanad Al-Hachamii 2 20.1 Naming Carboxylic Acids •Carboxylic Acids, RCO2H •If derived from open-chain alkanes, replace the terminal -e of the alkane name with -oic acid •The carboxyl carbon atom is C1 Based on McMurry, Organic Chemistry, Chapter 20, 6th Dr.Mohanad Al-Hachamii edition, (c) 2003 3 Alternative Names Compounds with CO2H bonded to a ring are named using the suffix -carboxylic acid The CO2H carbon is not itself numbered in this system Use common names for formic acid (HCOOH) and acetic acid (CH3COOH) – see Table 20.1 Based on McMurry, Organic Chemistry, Chapter 20, 6th edition, (c) 2003 Dr.Mohanad Al-Hachamii 4 5 • Greek letters are used to designate the location of substituents in common names. • The carbon adjacent to the COOH is called the carbon, followed by the carbon, followed by the carbon, the carbon and so forth down the chain. • The last carbon in the chain is sometimes called the carbon. • The carbon in the common system is numbered C2 in the IUPAC system. 6 • Compounds containing two carboxy groups are called diacids. Diacids are named using the suffix –dioic acid. -

Amidation of Carboxylic Acids with Amines by Nb2o5 As a Reusable Lewis Acid Catalyst
Title Amidation of Carboxylic Acids with Amines by Nb2O5 as a Reusable Lewis Acid Catalyst Author(s) Ali, Md. A.; Siddiki, S. M. A. H.; Onodera, Wataru; Kon, Kenichi; Shimizu, Ken-ichi ChemCatChem, 7(21), 3555-3561 Citation https://doi.org/10.1002/cctc.201500672 Issue Date 2015-11-02 Doc URL http://hdl.handle.net/2115/63409 This is the accepted version of the following article: ChemCatChem. 7, 3555-3561(2015), which has been published in Rights final form at http://onlinelibrary.wiley.com/doi/10.1002/cctc.201500672. Type article (author version) File Information amide-wi.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Amidation of Carboxylic Acids with Amines by Nb2O5 as Reusable Lewis Acid Catalyst Md. A. Ali,[a] S. M. A. H. Siddiki,[b] Wataru Onodera,[a] Kenichi Kon,[a] Ken-ichi Shimizu*[a,b] [a] Catalysis Research Center, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan [b] Elements Strategy Initiative for Catalysts and Batteries, Kyoto University, Katsura, Kyoto 615-8520, Japan *Corresponding author Ken-ichi Shimizu Catalysis Research Center, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan E-mail: [email protected] Abstract: Among 28 types of heterogeneous and homogenous catalysts tested, Nb2O5 shows the highest yield for direct amidation of n-dodecanoic acid with a less reactive amine (aniline). The catalytic amidation by Nb2O5 is applicable to a wide range of carboxylic acids and amines with various functional groups, and the catalyst is reusable. Comparison of the results of the catalytic study and infrared (IR) study of the acetic acid adsorbed on the catalyst suggests that activation of carbonyl group of the carboxylic acid by Lewis acid sites on Nb2O5 is responsible for the high activity of Nb2O5. -

Chemical Synthesis of Peptides and Peptide Thioesters Indrajeet Sharma Wayne State University
Wayne State University Wayne State University Dissertations 1-1-2011 Chemical Synthesis Of Peptides And Peptide Thioesters Indrajeet Sharma Wayne State University Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Part of the Organic Chemistry Commons Recommended Citation Sharma, Indrajeet, "Chemical Synthesis Of Peptides And Peptide Thioesters" (2011). Wayne State University Dissertations. Paper 205. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. CHEMICAL SYNTHESIS OF PEPTIDES AND PEPTIDE THIOESTERS by INDRAJEET SHARMA DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2011 MAJOR: CHEMISTRY (Organic) Approved by: Advisor Date DEDICATION This dissertation is dedicated to my family for their endless love and support. ii ACKNOWLEDGEMENTS First and foremost, I would like to express my deepest gratitude to my graduate advisor Professor David Crich for his advice and mentoring through all the years of my research work in his laboratories at the University of Illinois at Chicago and at Wayne State University. This work would not have reached its zenith without his unwavering guidance. He has been really supportive of my work not only with his remarkable knowledge and painstaking guidance, but also with his valuable suggestions and constant encouragement. I wish to thank my committee members Professor Zhongwu Guo, Professor Ashok S. Bhagwat, and Professor Steven J. Sucheck for their valuable time and suggestions to this dissertation and defense. -

Synthesis of New Linear Polymers Containing Thiocarbonyl Groups: Polyaddition of Dicarbothioic Acid to Diolefins
Polymer Journal, Vol. 26, No. 1, pp 49-59 (1994) Synthesis of New Linear Polymers Containing Thiocarbonyl Groups: Polyaddition of Dicarbothioic Acid to Diolefins Eiichi KOBAYASHI,* Kazuyoshi TENDO, Masato KATO, and Sadahito AoSHIMA Department of Industrial Chemistry, Faculty of Science and Technology, Science University of Tokyo, Noda, Chiba 278, Japan (Received May 24, 1993) ABSTRACT: The polyaddition of 1,4-benzenedicarbothioic acid to 1,4-divinylbenzene or 1,4-diisopropenylbenzene was carried out at 70°C in toluene under a nitrogen atmosphere by radical mode such as by addition of AIBN or UV irradiation. Both diolefin compounds gave polymers in a 90% yield. The polymer obtained from I ,4-diisopropenylbenzene was soluble in chloroform, and the molecular weight of the polymer (Mw) was 40000. On the other hand, the polymer obtained from 1,4-divinylbenzene was partly soluble in conventional organic solvents, possibly due to a cross linkage formation by a pendant vinyl group. The model addition reaction of thiobenzoic acid to styrene gave a by-product ca. 8% being the I : 2 adduct with respect to thiobenzoic acid and styrene. Alkali hydrolysis of both polymers was investigated to confirm the cross linkage structures in the polymers. KEY WORDS Polyaddition / Polythioester / Addition Reaction/ Hydrolysis / 1,4-Benzenedicarbothioic Acid / 1,4-Divinylbenzene / 1,4-Diisoprope nylbenzene / Thiobenzoic Acid/ Styrene/ oc-Methylstyrene / Addition reactions of carbothioic acids to (nb8 = 1.81) and exhibited the reversible phase olefins occur easily and are used to prepare transition by heat mode. various thioesters or polythioesters. For CH2=CH-<Q)--CH=CH2 + HS-<Q)--SH R or UV example, Ipatieff et al. -

REVIEW ARTICLE View Journal | View Issue
Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Developments and recent advancements in the field of endogenous amino acid selective bond Cite this: Chem. Soc. Rev., 2015, 44,5495 forming reactions for bioconjugation Oleksandr Konievab and Alain Wagner*a Bioconjugation methodologies have proven to play a central enabling role in the recent development of biotherapeutics and chemical biology approaches. Recent endeavours in these fields shed light on unprecedented chemical challenges to attain bioselectivity, biocompatibility, and biostability required by modern applications. In this review the current developments in various techniques of selective bond Received 19th January 2015 forming reactions of proteins and peptides were highlighted. The utility of each endogenous amino DOI: 10.1039/c5cs00048c acid-selective conjugation methodology in the fields of biology and protein science has been surveyed with emphasis on the most relevant among reported transformations; selectivity and practical use have www.rsc.org/chemsocrev been discussed. Creative Commons Attribution 3.0 Unported Licence. Contents 1.5 Histidine 1.5.1 Epoxides Introduction 1.5.2 Complexes with transition metals 1 In-chain conjugation 1.5.3 Michael addition 1.1 Lysine 1.5.4 Miscellaneous histidine-selective reagents 1.1.1 Isocyanates and isothiocyanates 1.6 Tyrosine This article is licensed under a 1.1.2 Activated esters 1.6.1 O-Derivatisation 1.1.3 Reductive amination of aldehydes 1.6.2 O-Oxidatiove coupling 1.1.4 Sulfonyl halides and sulfonates 1.6.3 Diazonium reagents Open Access Article. Published on 22 May 2015. Downloaded 10/8/2021 1:59:09 PM. -
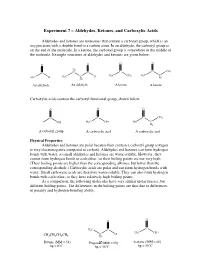
Experiment 7 – Aldehydes, Ketones, and Carboxylic Acids
Experiment 7 – Aldehydes, Ketones, and Carboxylic Acids Aldehydes and ketones are molecules that contain a carbonyl group, which is an oxygen atom with a double bond to a carbon atom. In an aldehyde, the carbonyl group is on the end of the molecule. In a ketone, the carbonyl group is somewhere in the middle of the molecule. Example structures of aldehydes and ketones are given below. O O O O H 2 C C CH C C C 3 H C C H H3C CH3 H3C C H3C H 3 H H2 2 An aldehyde An aldehyde A ketone A ketone Carboxylic acids contain the carboxyl functional group, shown below. O O O C C C CH3 OH H3C OH HO C H2 A carboxyl group A carboxylic acid A carboxylic acid Physical Properties Aldehydes and ketones are polar because they contain a carbonyl group (oxygen is very electronegative compared to carbon). Aldehydes and ketones can form hydrogen bonds with water, so small aldehydes and ketones are water-soluble. However, they cannot form hydrogen bonds to each other, so their boiling points are not very high. (Their boiling points are higher than the corresponding alkanes, but lower than the corresponding alcohols.) Carboxylic acids are polar and can form hydrogen bonds with water. Small carboxylic acids are therefore water-soluble. They can also form hydrogen bonds with each other, so they have relatively high boiling points. As a comparison, the following molecules have very similar molar masses, but different boiling points. The differences in the boiling points are thus due to differences in polarity and hydrogen-bonding ability. -

New Catalytic Transformations of Carboxylic Acids*
Pure Appl. Chem., Vol. 80, No. 8, pp. 1725–1733, 2008. doi:10.1351/pac200880081725 © 2008 IUPAC New catalytic transformations of carboxylic acids* Lukas J. Gooßen‡, Käthe Gooßen, Nuria Rodríguez, Mathieu Blanchot, Christophe Linder, and Bettina Zimmermann FB Chemie – Department of Organic Chemistry, Technical University Kaiserslautern, Erwin-Schrödinger-Str. Geb. 54, 67663 Kaiserslautern, Germany Abstract: A series of metal-catalyzed processes are presented, in which carboxylic acids act as sources of either carbon nucleophiles or electrophiles, depending on the catalyst em- ployed, the mode of activation, and the reaction conditions. A first reaction mode is the ad- dition of carboxylic acids or amides over C–C multiple bonds, giving rise to enol esters or enamides, respectively. The challenge here is to control both the regio- and stereoselectivity of these reactions by the choice of the catalyst system. Alternatively, carboxylic acids can ef- ficiently be decarboxylated using new Cu catalysts to give aryl-metal intermediates. Under protic conditions, these carbon nucleophiles give the corresponding arenes. If carboxylate salts are employed instead of the free acids, the aryl-metal species resulting from the catalytic decarboxylation can be utilized for the synthesis of biaryls in a novel cross-coupling reaction with aryl halides, thus replacing stoichiometric organometallic reagents. An activation with coupling reagents or simple conversion to esters allows the oxidative addition of carboxylic acids to transition-metal catalysts under formation of acyl-metal species, which can either be reduced to aldehydes, or coupled with nucleophiles. At elevated temperatures, such acyl- metal species decarbonylate, so that carboxylic acids become synthetic equivalents for aryl or alkyl halides, e.g., in Heck reactions. -

13. Carbonyl Compounds. Ketones, Aldehydes, and Carboxylic Acids 14
(7-9/94)(10/96)(06,9-11/04) Neuman Chapter 13 Chapter 13 Carbonyl Compounds: Ketones, Aldehydes, Carboxylic Acids from Organic Chemistry by Robert C. Neuman, Jr. Professor of Chemistry, emeritus University of California, Riverside [email protected] <http://web.chem.ucsb.edu/~neuman/orgchembyneuman/> Chapter Outline of the Book ************************************************************************************** I. Foundations 1. Organic Molecules and Chemical Bonding 2. Alkanes and Cycloalkanes 3. Haloalkanes, Alcohols, Ethers, and Amines 4. Stereochemistry 5. Organic Spectrometry II. Reactions, Mechanisms, Multiple Bonds 6. Organic Reactions *(Not yet Posted) 7. Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution 8. Alkenes and Alkynes 9. Formation of Alkenes and Alkynes. Elimination Reactions 10. Alkenes and Alkynes. Addition Reactions 11. Free Radical Addition and Substitution Reactions III. Conjugation, Electronic Effects, Carbonyl Groups 12. Conjugated and Aromatic Molecules 13. Carbonyl Compounds. Ketones, Aldehydes, and Carboxylic Acids 14. Substituent Effects 15. Carbonyl Compounds. Esters, Amides, and Related Molecules IV. Carbonyl and Pericyclic Reactions and Mechanisms 16. Carbonyl Compounds. Addition and Substitution Reactions 17. Oxidation and Reduction Reactions 18. Reactions of Enolate Ions and Enols 19. Cyclization and Pericyclic Reactions *(Not yet Posted) V. Bioorganic Compounds 20. Carbohydrates 21. Lipids 22. Peptides, Proteins, and α−Amino Acids 23. Nucleic Acids **************************************************************************************