Direct Synthesis of Amides and Imides by Using Heterogeneous Lewis Acid Catalysts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nomenclature of Carboxylic Acids • Select the Longest Carbon Chain Containing the Carboxyl Group
Chapter 5 Carboxylic Acids and Esters Carboxylic Acids • Carboxylic acids are weak organic acids which Chapter 5 contain the carboxyl group (RCO2H): Carboxylic Acids and Esters O C O H O RCOOH RCO2H Chapter Objectives: O condensed ways of • Learn to recognize the carboxylic acid, ester, and related functional groups. RCOH writing the carboxyl • Learn the IUPAC system for naming carboxylic acids and esters. group a carboxylic acid C H • Learn the important physical properties of the carboxylic acids and esters. • Learn the major chemical reaction of carboxylic acids and esters, and learn how to O predict the products of ester synthesis and hydrolysis reactions. the carboxyl group • Learn some of the important properties of condensation polymers, especially the polyesters. Mr. Kevin A. Boudreaux • The tart flavor of sour-tasting foods is often caused Angelo State University CHEM 2353 Fundamentals of Organic Chemistry by the presence of carboxylic acids. Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea 2 Nomenclature of Carboxylic Acids • Select the longest carbon chain containing the carboxyl group. The -e ending of the parent alkane name is replaced by the suffix -oic acid. • The carboxyl carbon is always numbered “1” but the number is not included in the name. • Name the substituents attached to the chain in the Nomenclature of usual way. • Aromatic carboxylic acids (i.e., with a CO2H Carboxylic Acids directly connected to a benzene ring) are named after the parent compound, benzoic acid. O C OH 3 -

Optimisation Du Métabolisme Énergétique Du Soufre Chez La Bactérie Hyperthermophile Aquifex Aeolicus
Aix-Marseille Université Ecole Doctorale des Sciences de la Vie et de la Santé Thèse de Doctorat d’Université Mention Microbiologie Clément AUSSIGNARGUES Optimisation du métabolisme énergétique du soufre chez la bactérie hyperthermophile Aquifex aeolicus Soutenue le 17 Décembre 2012 devant le jury: Pr. Frédéric BARRAS Dr. Laurent COURNAC Dr. Bruno FRANZETTI Dr. Marie-Thérèse GIUDICI-ORTICONI Dr. Anne GODFROY Dr. Marianne ILBERT Laboratoire de Bioénergétique et Ingénierie des Protéines Centre National de la Recherche Scientifique Aix-Marseille Université Ecole Doctorale des Sciences de la Vie et de la Santé Thèse de Doctorat d’Université Mention Microbiologie Clément AUSSIGNARGUES Optimisation du métabolisme énergétique du soufre chez la bactérie hyperthermophile Aquifex aeolicus Soutenue le 17 Décembre 2012 devant le jury: Pr. Frédéric BARRAS Dr. Laurent COURNAC Dr. Bruno FRANZETTI Dr. Marie-Thérèse GIUDICI-ORTICONI Dr. Anne GODFROY Dr. Marianne ILBERT Laboratoire de Bioénergétique et Ingénierie des Protéines Centre National de la Recherche Scientifique Sommaire Sommaire Introduction 2 Chapitre I- Métabolismes énergétiques du soufre 4 I) Le soufre, un élément essentiel aux multiples facettes 6 II) Le soufre utilisé comme composé énergétique 9 A) Oxydation des composés du soufre 10 1. Oxydation du sulfure d’hydrogène H2S 10 a) La Sulfure Quinone Oxydoréductase (SQR) 10 b) La flavocytochrome c sulfure déshydrogénase (FCSD) 13 2- 2. Oxydation du thiosulfate (S2O3 ) 14 a) Thiosulfate : accepteur oxydoréductase (TAOR) 14 b) Le système Sox 16 2- 3. Oxydation du sulfite (SO3 ) 20 a) Sulfite : accepteur oxydoréductase (SAOR) 21 b) Voie de l’APS 23 4. Oxydation du soufre stocké dans les globules 26 B) La réduction du soufre : soufre/polysulfure réductase 29 C) Un cas particulier : la dismutation du soufre (SOR) 33 1. -

And Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: a Review
catalysts Review Metal-Catalyzed Intra- and Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: A Review Javier Francos and Victorio Cadierno * Laboratorio de Compuestos Organometálicos y Catálisis (Unidad Asociada al CSIC), Centro de Innovación en Química Avanzada (ORFEO-CINQA), Departamento de Química Orgánica e Inorgánica, IUQOEM, Facultad de Química, Universidad de Oviedo, Julián Clavería 8, E-33006 Oviedo, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-985-103453 Received: 19 October 2017; Accepted: 2 November 2017; Published: 6 November 2017 Abstract: The metal-catalyzed addition of carboxylic acids to alkynes is a very effective tool for the synthesis of carboxylate-functionalized olefinic compounds in an atom-economical manner. Thus, a large variety of synthetically useful lactones and enol-esters can be accessed through the intra- or intermolecular versions of this process. In order to reduce the environmental impact of these reactions, considerable efforts have been devoted in recent years to the development of catalytic systems able to operate in aqueous media, which represent a real challenge taking into account the tendency of alkynes to undergo hydration in the presence of transition metals. Despite this, different Pd, Pt, Au, Cu and Ru catalysts capable of promoting the intra- and intermolecular addition of carboxylic acids to alkynes in a selective manner in aqueous environments have appeared in the literature. In this review article, an overview of this chemistry is provided. The synthesis of b-oxo esters by catalytic addition of carboxylic acids to terminal propargylic alcohols in water is also discussed. Keywords: aqueous catalysis; alkynes; carboxylic acids; alkynoic acids; hydrocarboxylation reactions; cycloisomerization reactions; enol esters; lactones; b-oxo esters 1. -

Synthesis of High Performance Phenolphthalein-Based Cardo Poly(Ether Ketone Imide)S Via Aromatic Nucleophilic Substitution Polymerization
Synthesis of high performance phenolphthalein-based cardo poly(ether ketone imide)s via aromatic nucleophilic substitution polymerization * * Jiangtao Liu, Guofei Chen , Junchao Guo, Nafeesa Mushtaq, Xingzhong Fang Ningbo Key Laboratory of Polymer Materials, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, Zhejiang 315201, China article info abstract Article history: A series of cardo poly(ether ketone imide)s (PEKI-C) containing large-volume bulky phthalide groups Received 13 January 2015 were prepared by aromatic nucleophilic substitution reaction of commercially available phenolphthalein Received in revised form and 4,40-bis(4-fluorophthalimido)diphenyl ether/4,40-difluorobenzophenone. The glass transition tem- 9 May 2015 peratures (T s) increased from 221 to 278 C with increasing the content of 4,40-bis(4-fluorophthalimido) Accepted 29 May 2015 g diphenyl ether moiety in the copolymerization. The 5% weight loss temperatures (T ) of PEKI-C (aee) Available online 5 June 2015 5% reached up to 472e495 C in nitrogen and 466e481 C in air. Flexible films, which could be easily cast from the polymer solutions, exhibited good mechanical properties with tensile strengths of 73e124 MPa, Keywords: e e Phenolphthalein elongations at break of 9.7 12.8%, and tensile moduli of 2.2 2.8 GPa. Their films with cut-off wave- Cardo poly(ether ketone imide)s lengths from 336 to 368 nm were transparent and essentially colorless. It is noted that the Tg, T5%, tensile Aromatic nucleophilic substitution strengths, elongations at break, and tensile moduli of PEKI-C (aee) increased with increasing the content 0 polymerization of 4,4 -bis(4-fluorophthalimido)diphenyl ether in the copolymerization. -

Recent Advances and Future Prospects of Phthalimide Derivatives
Journal of Applied Pharmaceutical Science Vol. 6 (03), pp. 159-171, March, 2016 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2016.60330 ISSN 2231-3354 Recent Advances and Future Prospects of Phthalimide Derivatives Neelottama Kushwahaa*, Darpan Kaushikb aPranveer Singh Institute of Technology, Kanpur, India. bPrasad Institute of Technology, Jaunpur, India. ABSTRACT ARTICLE INFO Article history: Among bicyclic non-aromatic nitrogen heterocycles, phthalimides are an interesting class of compounds with a Received on: 21/05/2015 large range of applications. Phthalimide contains an imide functional group and may be considered as nitrogen Revised on: 13/09/2015 analogues of anhydrides or as diacyl derivatives of ammonia. They are lipophilic and neutral compounds and can Accepted on: 02/11/2015 therefore easily cross biological membranes in vivo and showing different pharmacological activities. In the Available online: 10/03/2016 present work compounds containing phthalimide subunit have been described as a scaffold to design new prototypes drug candidates with different biological activities and are used in different diseases as, for example Key words: AIDS, tumor, diabetes, multiple myeloma, convulsion, inflammation, pain, bacterial infection among others. Phthalimide derivatives, imide, biological activity. INTRODUCTION have served as starting materials and intermediates for the synthesis of many types of alkaloids and pharmacophores. Phthalimides possess a structural feature –CO-N(R)- CO- and an imide ring which help them to be biologically active Structure of Phthalimide and pharmaceutically useful. Phthalimides have received attention due to their androgen receptor antagonists (Sharma et Phthalimide is an imido derivative of phthalic acid. In al., 2012), anticonvulsant (Kathuria and Pathak, 2012), organic chemistry, imide is a functional group consisting of two antimicrobial (Khidre et al., 2011), hypoglycaemic (Mbarki and carbonyl groups bound to nitrogen. -

United States Patent Office Patented Sept
3,274,211 United States Patent Office Patented Sept. 20, 1966 2 cording to the process of this invention. Other ketones 3,274,211 N-ALKYLATION OF AMDES AND MEDES which may be utilized to give particular N-alkylation Louis Schmerling, Riverside, III., assignor to Universal products include methyl ethyl ketone, methyl propyl Oil Products Company, Des Plaines, Ill., a corporation ketone, methylbutyl ketone, methyl amyl ketone, methyl of Delaware hexyl ketone, methyl heptyl ketone, methyl octyl ketone, No Drawing. Filed Feb. 4, 1964, Ser. No. 342,549 etc., ethyl ethyl ketone, ethyl propyl ketone, ethyl butyl 9 Claims. (Cl. 260-326) ketone, ethyl amyl ketone, ethylhexyl ketone, ethyl hep tyl ketone, etc., propyl propyl ketone, propylbutyl ketone, This invention relates to a novel process for the N propyl amyl ketone, propylhexyl ketone, etc., butylbutyl alkylation of carboxylic acid amides and imides. More ketone, butyl amyl ketone, butyl hexyl ketone, etc. particularly, this invention relates to the reductive alkyla Other suitable but not necessarily equivalent ketones com tion of carboxylic acid amides and imides to give N prise cyclic ketones including cyclohexanone, benzophe alkylation products. none, etc., alkyl aryl ketones, alkyl cyclohexyl ketones, The alkylation products herein contemplated are par aryl aryl ketones, aryl cyclohexyl ketones, etc., including ticularly adapted to the preparation of primary amines. 15 compounds like acetophenone, ethyl phenylketone, propyl For example, N-isobutylacetamide, the reductive alkyla phenyl ketone, butyl phenyl ketone, amyl phenyl ketone, tion product of acetamide and isobutyraldehyde, is hydro etc., methyl cyclohexyl ketone, ethyl cyclohexyl ketone, lyzed to isobutylamine and acetic acid, the latter being propyl cyclohexyl ketone, butyl cyclohexyl ketone, etc. -

Carboxylic Acid Addition to Terminal Alkynes Utilizing Ammonium Tagged
Journal of Organometallic Chemistry 883 (2019) 11e16 Contents lists available at ScienceDirect Journal of Organometallic Chemistry journal homepage: www.elsevier.com/locate/jorganchem Carboxylic acid addition to terminal alkynes utilizing ammonium tagged Hoveyda-Grubbs catalyst supported on magnetically separable core/shell silica: A highly reusable and air compatible catalytic system € € * ** Bengi Ozgün Oztürk , Didar Gürcü, Solmaz Karabulut S¸ ehitoglu Hacettepe University, Faculty of Science, Chemistry Department, 06800, Beytepe, Ankara, Turkey article info abstract Article history: In this study, the performance of ammonium tagged Hoveyda-Grubbs catalyst supported on magnetically Received 24 October 2018 separable core/shell silica gel was tested on carboxylic acid addition reactions to terminal alkynes using a Received in revised form variety of carboxylic acid derivatives under air atmosphere. The catalytic system was found to be 13 December 2018 compatible with air atmosphere and can tolerate even non-degassed solvents. The reaction parameters Accepted 10 January 2019 such as temperature, substrate/catalyst ratio and the effect of carboxylic acid on the selectivity and yield Available online 14 January 2019 of the reaction were investigated in details. The reaction of arylacetylenes with acetic acid yielded the corresponding E-isomer with conversion values up to 99% with a catalytic loading of 1% Ru. The reus- Keywords: Carboxylic acid ability of the catalyst was tested using acetic acid/benzoic acid and phenylacetylene in toluene at 85 C Alkyne under air atmosphere. The catalyst was found to be highly reusable and maintained its activity up to 11th Ruthenium run, reaching a conversion value of 83% with minimum ruthenium leaching. Reusable catalyst © 2019 Elsevier B.V. -
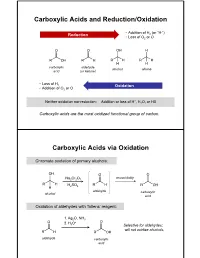
Carboxylic Acids and Reduction/Oxidation Carboxylic
Carboxylic Acids and Reduction/Oxidation • Addition of H (or “H-”) Reduction 2 • Loss of O2 or O carboxylic aldehyde alcohol alkane acid (or ketone) • Loss of H 2 Oxidation • Addition of O2 or O + Neither oxidation nor reduction: Addition or loss of H , H2O, or HX Carboxylic acids are the most oxidized functional group of carbon. Carboxylic Acids via Oxidation Chromate oxidation of primary alcohols: unavoidably Na2Cr2O7 H2SO4 aldehyde carboxylic alcohol acid Oxidation of aldehydes with Tollens’ reagent: 1. Ag2O, NH3 2. H O+ 3 Selective for aldehydes; will not oxidize alcohols. aldehyde carboxylic acid Carboxylic Acids via Oxidation Oxidative cleavage of alkynes: 1. O3 2. H2O internal alkyne Oxidation of benzylic carbons: KMnO4 Purifying Carboxylic Acids by Acid-Base Extraction Acidic and basic organic molecules can be separated from other substances by manipulating their protonation state and solubility. Let’s say we run a reaction. Na2Cr2O7 + unreacted H2SO4 + Na+ 3+ We want this… + Cr(H2O)6 + organic + HSO - …but not these. side products 4 How do we isolate our desired product from the other materials, without using distillation or chromatography? Acid-Base Extraction Step 1: Remove inorganics from organics via differential solubility. a separatory (sept) funnel - Na+ HSO4 Cr(H O) 3+ H2O 2 6 CHCl3 + organic side densities: products (H2O) = 1 g/mL (CHCl3) = 1.48 g/mL A large density difference ensures the two liquids separate completely and quickly. (Much faster than, say, oil and water.) Step 2: Add basic water to deprotonate carboxylic acid, and transfer it to the aqueous phase. - Na+ HSO4 3+ discard Cr(H2O)6 H2O layer + organic + organic side side products products add discard NaOH/ H2O CHCl3 layer + organic side products Step 3: Re-acidify carboxylate to return it to organic solution. -

Green Radicals of Potassium Poly(Heptazine Imide) Using Light and Benzylamine† Cite This: J
Journal of Materials Chemistry A View Article Online COMMUNICATION View Journal | View Issue Green radicals of potassium poly(heptazine imide) using light and benzylamine† Cite this: J. Mater. Chem. A,2019,7, 24771 Yevheniia Markushyna,‡a Paolo Lamagni,‡abc Christian Teutloff,d c bc a a Received 28th August 2019 Jacopo Catalano, Nina Lock,* Guigang Zhang, Markus Antonietti a Accepted 7th October 2019 and Aleksandr Savateev * DOI: 10.1039/c9ta09500d rsc.li/materials-a Tinted long-lived ionic carbon nitride radicals were recently intro- poly(heptazine imides) are capable of photocharging.8 Fig. 1 duced and applied in photocatalysis and energy storage. However, the sketches the mechanism of IDEAS (Illumination-Driven reason for their higher activity in the photocatalytic reaction and Electron Accumulation in Semiconductors) – acapacious optimal conditions for generating such radicals remain vague. Herein, acronym that we propose for this process in the present Creative Commons Attribution 3.0 Unported Licence. we study the conditions for carbon nitride photocharging to achieve communication. a higher charge density and validate a convenient method to quantify On the energy scale, it can be schematically explained as the number of electrons accumulated in carbon nitride semi- follows. By absorbing a photon, potassium poly(heptazine conductors by quenching its radicals with methylviologen in the dark. imide) (K-PHI) is converted to the excited state (K-PHI*). Elec- In the presence of CO2, potassium poly(heptazine imide) (K-PHI) can tron transfer from the HOMO of the electron donor (ED) lying be charged by up to 1000 mmol of electrons per gram of the material above the valence band (VB) of K-PHI reduces K-PHI* and gives À using benzylamine as an electron donor. -

Chapter 20: Carboxylic Acids and Nitriles
CHAPTER 20 CARBOXYLIC ACIDS Based on McMurry’s Organic Chemistry, 6th edition Dr.Mohanad Al-Hachamii The Importance of Carboxylic Acids (RCO2H) Starting materials for acyl derivatives (esters, amides, and acid chlorides) Abundant in nature from oxidation of aldehydes and alcohols in metabolism Acetic acid, CH3CO2H, - vinegar Butanoic acid, CH3CH2CH2CO2H (rancid butter) Long-chain aliphatic acids from the breakdown of fats Based on McMurry, Organic Chemistry, Chapter 20, 6th edition, (c) 2003 Dr.Mohanad Al-Hachamii 2 20.1 Naming Carboxylic Acids •Carboxylic Acids, RCO2H •If derived from open-chain alkanes, replace the terminal -e of the alkane name with -oic acid •The carboxyl carbon atom is C1 Based on McMurry, Organic Chemistry, Chapter 20, 6th Dr.Mohanad Al-Hachamii edition, (c) 2003 3 Alternative Names Compounds with CO2H bonded to a ring are named using the suffix -carboxylic acid The CO2H carbon is not itself numbered in this system Use common names for formic acid (HCOOH) and acetic acid (CH3COOH) – see Table 20.1 Based on McMurry, Organic Chemistry, Chapter 20, 6th edition, (c) 2003 Dr.Mohanad Al-Hachamii 4 5 • Greek letters are used to designate the location of substituents in common names. • The carbon adjacent to the COOH is called the carbon, followed by the carbon, followed by the carbon, the carbon and so forth down the chain. • The last carbon in the chain is sometimes called the carbon. • The carbon in the common system is numbered C2 in the IUPAC system. 6 • Compounds containing two carboxy groups are called diacids. Diacids are named using the suffix –dioic acid. -

Amidation of Carboxylic Acids with Amines by Nb2o5 As a Reusable Lewis Acid Catalyst
Title Amidation of Carboxylic Acids with Amines by Nb2O5 as a Reusable Lewis Acid Catalyst Author(s) Ali, Md. A.; Siddiki, S. M. A. H.; Onodera, Wataru; Kon, Kenichi; Shimizu, Ken-ichi ChemCatChem, 7(21), 3555-3561 Citation https://doi.org/10.1002/cctc.201500672 Issue Date 2015-11-02 Doc URL http://hdl.handle.net/2115/63409 This is the accepted version of the following article: ChemCatChem. 7, 3555-3561(2015), which has been published in Rights final form at http://onlinelibrary.wiley.com/doi/10.1002/cctc.201500672. Type article (author version) File Information amide-wi.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Amidation of Carboxylic Acids with Amines by Nb2O5 as Reusable Lewis Acid Catalyst Md. A. Ali,[a] S. M. A. H. Siddiki,[b] Wataru Onodera,[a] Kenichi Kon,[a] Ken-ichi Shimizu*[a,b] [a] Catalysis Research Center, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan [b] Elements Strategy Initiative for Catalysts and Batteries, Kyoto University, Katsura, Kyoto 615-8520, Japan *Corresponding author Ken-ichi Shimizu Catalysis Research Center, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan E-mail: [email protected] Abstract: Among 28 types of heterogeneous and homogenous catalysts tested, Nb2O5 shows the highest yield for direct amidation of n-dodecanoic acid with a less reactive amine (aniline). The catalytic amidation by Nb2O5 is applicable to a wide range of carboxylic acids and amines with various functional groups, and the catalyst is reusable. Comparison of the results of the catalytic study and infrared (IR) study of the acetic acid adsorbed on the catalyst suggests that activation of carbonyl group of the carboxylic acid by Lewis acid sites on Nb2O5 is responsible for the high activity of Nb2O5. -

Chemical Synthesis of Peptides and Peptide Thioesters Indrajeet Sharma Wayne State University
Wayne State University Wayne State University Dissertations 1-1-2011 Chemical Synthesis Of Peptides And Peptide Thioesters Indrajeet Sharma Wayne State University Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Part of the Organic Chemistry Commons Recommended Citation Sharma, Indrajeet, "Chemical Synthesis Of Peptides And Peptide Thioesters" (2011). Wayne State University Dissertations. Paper 205. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. CHEMICAL SYNTHESIS OF PEPTIDES AND PEPTIDE THIOESTERS by INDRAJEET SHARMA DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2011 MAJOR: CHEMISTRY (Organic) Approved by: Advisor Date DEDICATION This dissertation is dedicated to my family for their endless love and support. ii ACKNOWLEDGEMENTS First and foremost, I would like to express my deepest gratitude to my graduate advisor Professor David Crich for his advice and mentoring through all the years of my research work in his laboratories at the University of Illinois at Chicago and at Wayne State University. This work would not have reached its zenith without his unwavering guidance. He has been really supportive of my work not only with his remarkable knowledge and painstaking guidance, but also with his valuable suggestions and constant encouragement. I wish to thank my committee members Professor Zhongwu Guo, Professor Ashok S. Bhagwat, and Professor Steven J. Sucheck for their valuable time and suggestions to this dissertation and defense.