2006/2007 South Africa Yearbook: 21
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Botshabelo and Thaba Nchu CRDP
Kopanong Ward # 41602001 Comprehensive Rural Development Program Status Quo Report CHIEF DIRECTORATE: SPATIAL PLANNING AND INFORMATION July 25, 2011 Authored by: SPI Free State TABLE OF CONTENTS 1. INTRODUCTION ........................................................................................................ 3 1.2. OBJECTIVE OF THE STUDY ........................................................................................... 3 1.3. BACKGROUND ........................................................................................................ 3 2. RESEARCH DESIGN ..................................................................................................... 4 2.1. PROBLEM STATEMENT ....................................................................................... 4 2.2. METHODOLOGY .............................................................................................. 5 2.2.1. DEFINITION OF RURAL AREAS (OECD) ............................................................... 7 3. STUDY AREA .............................................................................................................. 8 3.1. PROVINCIAL CONTEXT ...................................................................................... 8 3.2. DISTRICT CONTEXT .......................................................................................... 9 3.3. LOCAL CONTEXT ........................................................................................... 10 3.4. PILOT SITE .................................................................................................. -

Death by Smallpox in 18Th and 19Th C. South Africa
Anistoriton Journal, vol. 11 (2007) Essay Section Death by smallpox investigating the relationship between anaemia and viruses in 18th and 19th century South Africa Tanya R. Peckmann, Ph.D. Saint Mary's University, Canada The historical record combined with the presence of large numbers of individuals exhibiting skeletal responses to anaemia (porotic hyperostosis and cribra orbitalia; PH and CO) are the main reasons for investigating the presence of smallpox in three South African communities, Griqua, Khoe, and ‘Black’ African, during the 18th and 19th centuries. The smallpox virus (variola) raged throughout South Africa every twenty or thirty years during the eighteenth and nineteenth centuries and was responsible for the destruction of entire communities. It has an 80 to 90 per cent fatality rate among non-immune populations (Aufderheide & Rodríguez-Martín 1998; Young 1998) and all ages are susceptible. The variola virus can only survive in densely populated areas and therefore sedentary communities, such as those present in agricultural and pastoral based societies, are more susceptible to acquiring the disease. Smallpox may remodel bone in the form of osteomyelitis variolosa (‘smallpox arthritis’) (Aufderheide & Rodríguez-Martín 1998; Jackes 1983; Ortner & Putschar 1985) which causes the reduction of longitudinal bone growth (Jackes 1983). However, since smallpox only remodels bone in very few individuals and solely in children the only method for unconditionally determining the presence of the smallpox virus in a skeletal population is by performing DNA and PCR analyses. Survival from smallpox affords the individual natural immunity for the remainder of their life. The virus is undetectable in a smallpox survivor as they will possess the antibodies for the disease and therefore will have gained natural immunity for the remainder of his or her life. -

Thesis Hum 2007 Mcdonald J.Pdf
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town 'When Shall These Dry Bones Live?' Interactions between the London Missionary Society and the San Along the Cape's North-Eastern Frontier, 1790-1833. Jared McDonald MCDJAR001 A dissertation submitted in fulfillment of the requirements for the award of the degree of Master of Arts in Historical Studies Faculty of the Humanities University of Cape Town 2007 University of Cape Town COMPULSORY DECLARATION This work has not been previously submitted in whole, or in part, for the award of any degree. It is my own work. Each significant contribution to, and quotation in, this dissertation from the work, or works, of other people has been attributed, and has been cited and referenced. Signature:_fu""""',_..,"<_l_'"'"-_(......\b __ ~_L ___ _ Date: 7 September 2007 "And the Lord said to me, Prophe,\y to these bones, and scry to them, o dry bones. hear the Word ofthe Lord!" Ezekiel 37:4 Town "The Bushmen have remained in greater numbers at this station ... They attend regularly to hear the Word of God but as yet none have experiencedCape the saving effects ofthe Gospe/. When shall these dry bones oflive? Lord thou knowest. -

Xhariep Magisterial District
!. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. XXhhaarriieepp MMaaggiisstteerriiaall DDiissttrriicctt !. !. !. !. !. !. !. !. !. !. TheunissenS ubD istrict !. BARKLY WEST R59 R707 !. ST DEALESVILLE R708 SAPS WINBURG ST R70 !. R370 Lejwelepuitsa SAPS Dealesville R73 Winburg ST ST ST R31 Lejwelepuitsa ST Marquard !. ST LKN12 Boshof !. BRANDFORT Brandfort SAPS !. Soutpan R703 SAPS !. R64 STR64 Magiisteriiall R703 !. ST Sub District Marquard Senekal CAMPBELL ST Kimberley Dealesville ST Ficksburg !. !. !. !. R64 Sub BOSHOF SOUTPAN SAPS KIMBERLEY ST Sub !. !. !. SAPS Diistriict R64 SAPS Brandfort !. SAPS ST R709 Sub District District Sub !. !. Sub ST District Verkeerdevlei MARQUARD Sub District N1 !. SAPS Clocolan !. !. District STR700 KL District -

Kai ! Garib Final IDP 2020 2021
KAI !GARIB MUNICIPALITY Integrated Development Plan 2020/2021 “Creating an economically viable and fully developed municipality, which enhances the standard of living of all the inhabitants / community of Kai !Garib through good governance, excellent service delivery and sustainable development.” June 2020 TABLE OF CONTENTS FOREWORD.................................................................................................................1 2. IDP PLANNING PROCESS:......................................................................................2 2.1 IDP Steering Committee:...........................................................................................3 2.2 IDP Representative Forum.........................................................................................3 2.3 Process Overview: Steps & Events:.............................................................................4 2.4 Legislative Framework:…………………………………………………………………………………………...6 3. THE ORGANISATION:............................................................................................15 3.1 Institutional Development………………………………………………………………………………..... 15 3.2 The Vision & Mission:...............................................................................................16 3.3 The Values of Kai !Garib Municipality which guides daily conduct ...............................16 3.4 The functioning of the municipality............................................................................16 3.4.1 Council and council committees..............................................................................16 -
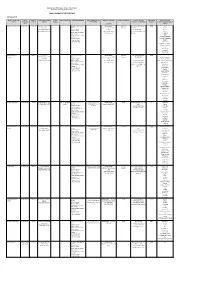
Public Libraries in the Free State
Department of Sport, Arts, Culture & Recreation Directorate Library and Archive Services PUBLIC LIBRARIES IN THE FREE STATE MOTHEO DISTRICT NAME OF FRONTLINE TYPE OF LEVEL OF TOWN/STREET/STREET STAND GPS COORDINATES SERVICES RENDERED SPECIAL SERVICES AND SERVICE STANDARDS POPULATION SERVED CONTACT DETAILS REGISTERED PERIODICALS AND OFFICE FRONTLINE SERVICE NUMBER NUMBER PROGRAMMES CENTER/OFFICE MANAGER MEMBERS NEWSPAPERS AVAILABLE IN OFFICE LIBRARY: (CHARTER) Bainsvlei Public Library Public Library Library Boerneef Street, P O Information and Reference Library hours: 446 142 Ms K Niewoudt Tel: (051) 5525 Car SA Box 37352, Services Ma-Tue, Thu-Fri: 10:00- (Metro) 446-3180 Fair Lady LANGENHOVENPARK, Outreach Services 17:00 Fax: (051) 446-1997 Finesse BLOEMFONTEIN, 9330 Electronic Books Wed: 10:00-18:00 karien.nieuwoudt@mangau Hoezit Government Info Services Sat: 8:30-12:00 ng.co.za Huisgenoot Study Facilities Prescribed books of tertiary Idees Institutions Landbouweekblad Computer Services: National Geographic Internet Access Rapport Word Processing Rooi Rose SA Garden and Home SA Sports Illustrated Sarie The New Age Volksblad Your Family Bloemfontein City Public Library Library c/o 64 Charles Information and Reference Library hours: 443 142 Ms Mpumie Mnyanda 6489 Library Street/West Burger St, P Services Ma-Tue, Thu-Fri: 10:00- (Metro) 051 405 8583 Africa Geographic O Box 1029, Outreach Services 17:00 Architect and Builder BLOEMFONTEIN, 9300 Electronic Books Wed: 10:00-18:00 Tel: (051) 405-8583 Better Homes and Garden n Government Info -

Federal Register/Vol. 74, No. 142/Monday, July 27, 2009/Notices
37000 Federal Register / Vol. 74, No. 142 / Monday, July 27, 2009 / Notices Africa from which citrus fruit is Inspection Service has determined the contact Dr. Patricia L. Foley, Center for authorized for importation into the regulatory review period for NAHVAX® Veterinary Biologics, Policy Evaluation United States, our review of the Marek’s Disease Vaccine and is and Licensing, VS, APHIS, 510 South information presented by the Republic publishing this notice of that 17th Street, Suite 104, Ames, IA 50010; of South Africa in support of its request determination as required by law. We phone (515) 232–5785, fax (515) 232– is examined in a commodity import have made this determination in 7120. evaluation document (CIED) titled response to the submission of an SUPPLEMENTARY INFORMATION: The ‘‘Recognition of Additional Magisterial application to the Commissioner of provisions of 35 U.S.C. 156, ’’ Extension Districts as Citrus Black Spot Pest-Free Patents and Trademarks, Department of of patent term,’’ provide, generally, that Areas for the Republic of South Africa.’’ Commerce, for the extension of a patent a patent for a product may be extended The CIED may be viewed on the that claims that veterinary biologic. for a period of up to 5 years as long as Regulations.gov Web site or in our DATES: We will consider all requests for the patent claims a product that, among reading room (see ADDRESSES above for revision of the regulatory review period instructions for accessing other things, was subject to a regulatory determination that we receive on or review period before its commercial Regulations.gov and information on the before August 26, 2009. -

Scientists Brave SA's Mightiest River to Kayak from Source To
Aquatic ecosystems The Orange River forms a green artery of life through the harsh and arid desert along the border of South Africa and Namibia. Courtesy Senqu2SeaCourtesy team Scientists brave SA’s mightiest river to kayak from source to sea When Irrigation Department Director, hile not as substantial to undertake rare extensive field Dr Alfred Dale Lewis, explored the lower as its cousin, the research. “The Orange is the iconic Zambezi, to the north, South African river – long, ancient reaches of the Orange River in December SouthW Africa’s largest river has and traversing varied and incredibly 1913 he walked most of the 400 km-long always captured the imagination of beautiful scenery, from grass moun- journey in one of the hottest years on those who gazed upon it. Local Khoi tain highlands to rocky desert. We named it the Gariep, meaning ‘big wanted to spend an extended period record. Now nearly a century later, three water’ or ‘great river’, while the San’s in nature, experiencing a long rather young researchers of the University of name for it meant ‘Dragon River’. It than a technically difficult adven- Cape Town (UCT) have completed a similar was European commander, Colonel ture,” explains the team. Robert Gordon, who gave the river adventure, traversing South Africa’s its ‘royal’ name, naming the river VALUABLE RESEARCH mightiest river in kayaks from its source after Dutch ruler, Prince William of in the Lesotho mountains to its mouth on Orange, 300 years ago. hile enjoying the scenery For Masters graduate Sam Jack, Wthe team also took time to the West Coast of South Africa. -

An Anthropological Study of Itinerancy and Domestic Fluidity Amongst the Karretjie People of the South African Karoo
CHILDHOOD: AN ANTHROPOLOGICAL STUDY OF ITINERANCY AND DOMESTIC FLUIDITY AMONGST THE KARRETJIE PEOPLE OF THE SOUTH AFRICAN KAROO by SARAH ADRIANA STEYN submitted in fulfilment of the requirements for the degree of MASTER OF ARTS in the subject ANTHROPOLOGY at the UNIVERSITY OF SOUTH AFRICA SUPERVISOR: PROFESSOR M. DE JONGH MARCH 2009 Dedicated to my children, Luke and Sarah-Anne In memory of my siblings, Doria and Francois I declare that Childhood: An Anthropological Study of Itinerancy and Domestic Fluidity Amongst the Karretjie People of the South African Karoo is my own work and that all the sources that I have used or quoted have been indicated and acknowledged by means of complete references. S A Steyn 2009.03.05 “The only difference between them and us is that we are ready to learn from them but they are not ready to learn from us ... in everyday life they must come to recognise us, respect us, value us ... for what we are. Only then will each one of us be able to discover the other”. (Words of a Roma delegate at a Conference organised by the Centre for Gypsy Research at the Université René Descartes, Paris, 1993) ABSTRACT The Karretjie People, or Cart People are a peripatetic community and are descendants of the KhoeKhoen and San, the earliest inhabitants of the Karoo region in South Africa. As a landless and disempowered community they are dependent upon others for food and other basic necessities specifically, and other resources generally. Compared to children in South Africa generally, the Karretjie children are in every sense of the most severely deprived. -

Review of Existing Infrastructure in the Orange River Catchment
Study Name: Orange River Integrated Water Resources Management Plan Report Title: Review of Existing Infrastructure in the Orange River Catchment Submitted By: WRP Consulting Engineers, Jeffares and Green, Sechaba Consulting, WCE Pty Ltd, Water Surveys Botswana (Pty) Ltd Authors: A Jeleni, H Mare Date of Issue: November 2007 Distribution: Botswana: DWA: 2 copies (Katai, Setloboko) Lesotho: Commissioner of Water: 2 copies (Ramosoeu, Nthathakane) Namibia: MAWRD: 2 copies (Amakali) South Africa: DWAF: 2 copies (Pyke, van Niekerk) GTZ: 2 copies (Vogel, Mpho) Reports: Review of Existing Infrastructure in the Orange River Catchment Review of Surface Hydrology in the Orange River Catchment Flood Management Evaluation of the Orange River Review of Groundwater Resources in the Orange River Catchment Environmental Considerations Pertaining to the Orange River Summary of Water Requirements from the Orange River Water Quality in the Orange River Demographic and Economic Activity in the four Orange Basin States Current Analytical Methods and Technical Capacity of the four Orange Basin States Institutional Structures in the four Orange Basin States Legislation and Legal Issues Surrounding the Orange River Catchment Summary Report TABLE OF CONTENTS 1 INTRODUCTION ..................................................................................................................... 6 1.1 General ......................................................................................................................... 6 1.2 Objective of the study ................................................................................................ -

Orange River Project
ORANGE RIVER PROJECT: OvERVIEW South Africa NAMIBIA BOTSWANA Orange-Senqu River Basin Vanderkloof Dam LESOTHO Gariep Dam LOCATION SOUTH AFRICA The Orange River Project (ORP) is the largest scheme in the Orange–Senqu River basin, and includes the two largest dams in South Africa, the Gariep and Vanderkloof. They regulate flows to the Orange River and increase assurance of supply. DESCRIPTION Gariep and Vanderkloof dams were constructed as part of the project, and have a combined storage of 8,500 million m3. The ORP includes several sub-systems. The Orange–Riet Water Scheme.* The Orange–Fish Transfer Tunnel.* The Orange–Vaal Transfer Scheme.* Bloem Water: Pipeline network between Gariep Dam and the towns of Trompsburg, Springfontein and Philippolis. Irrigation abstractions: Between Gariep Dam and downstream of Vanderkloof Dam, up to the confluence of the Vaal and Orange rivers (near the town of Marksdrift). Urban and industrial abstractions: Between Gariep Dam and Marksdrift (including Hopetown and Vanderkloof towns). Support to the Lower Orange Water Management Area Schemes: * Support to most of the demands in the Gariep Dam (© Hendrik van den Berg/Panoramio.com) Lower Orange, including irrigation, urban use and power generation. Caledon–Bloemfontein Government Water Scheme.* * Further details are given on separate pages PURPOSE The purpose of this very complex scheme is to supply demands within several sub-systems, including the Upper and Lower Orange water management areas all the way down to the Orange River mouth, and the Eastern Cape Province. These demands include irrigation, urban, industrial and environmental water requirements. Power generation is also part of the system, including at Gariep and Vanderkloof dams, which contributes to the Eskom national power grid. -

Eskom Audited Annual Results Presentation for the Year Ended 31 March 2011
Eskom Audited Annual Results Presentation for the year ended 31 March 2011 Select Committee on Labour and Public Enterprises 9 May 2012 Disclaimer This presentation does not constitute or form part of and should not be construed as, an offer to sell, or the solicitation or invitation of any offer to buy or subscribe for or underwrite or otherwise acquire, securities of Eskom Holdings Limited (“Eskom”), any holding company or any of its subsidiaries in any jurisdiction or any other person, nor an inducement to enter into any investment activity. No part of this presentation, nor the fact of its distribution, should form the basis of, or be relied on in connection with, any contract or commitment or investment decision whatsoever. This presentation does not constitute a recommendation regarding any securities of Eskom or any other person. Certain statements in this presentation regarding Eskom’s business operations may constitute “forward looking statements.” All statements other than statements of historical fact included in this presentation, including, without limitation, those regarding the financial position, business strategy, management plans and objectives for future operations of Eskom are forward looking statements. Forward-looking statements are not intended to be a guarantee of future results, but instead constitute Eskom’s current expectations based on reasonable assumptions. Forecasted financial information is based on certain material assumptions. These assumptions include, but are not limited to continued normal levels of operating performance and electricity demand in the Distribution and Transmission divisions and operational performance in the Generation and Primary Energy divisions consistent with historical levels, and incremental capacity additions through our Group Capital division at investment levels and rates of return consistent with prior experience, as well as achievements of planned productivity improvements throughout our business activities.