Federal Register/Vol. 74, No. 142/Monday, July 27, 2009/Notices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Botshabelo and Thaba Nchu CRDP
Kopanong Ward # 41602001 Comprehensive Rural Development Program Status Quo Report CHIEF DIRECTORATE: SPATIAL PLANNING AND INFORMATION July 25, 2011 Authored by: SPI Free State TABLE OF CONTENTS 1. INTRODUCTION ........................................................................................................ 3 1.2. OBJECTIVE OF THE STUDY ........................................................................................... 3 1.3. BACKGROUND ........................................................................................................ 3 2. RESEARCH DESIGN ..................................................................................................... 4 2.1. PROBLEM STATEMENT ....................................................................................... 4 2.2. METHODOLOGY .............................................................................................. 5 2.2.1. DEFINITION OF RURAL AREAS (OECD) ............................................................... 7 3. STUDY AREA .............................................................................................................. 8 3.1. PROVINCIAL CONTEXT ...................................................................................... 8 3.2. DISTRICT CONTEXT .......................................................................................... 9 3.3. LOCAL CONTEXT ........................................................................................... 10 3.4. PILOT SITE .................................................................................................. -

Death by Smallpox in 18Th and 19Th C. South Africa
Anistoriton Journal, vol. 11 (2007) Essay Section Death by smallpox investigating the relationship between anaemia and viruses in 18th and 19th century South Africa Tanya R. Peckmann, Ph.D. Saint Mary's University, Canada The historical record combined with the presence of large numbers of individuals exhibiting skeletal responses to anaemia (porotic hyperostosis and cribra orbitalia; PH and CO) are the main reasons for investigating the presence of smallpox in three South African communities, Griqua, Khoe, and ‘Black’ African, during the 18th and 19th centuries. The smallpox virus (variola) raged throughout South Africa every twenty or thirty years during the eighteenth and nineteenth centuries and was responsible for the destruction of entire communities. It has an 80 to 90 per cent fatality rate among non-immune populations (Aufderheide & Rodríguez-Martín 1998; Young 1998) and all ages are susceptible. The variola virus can only survive in densely populated areas and therefore sedentary communities, such as those present in agricultural and pastoral based societies, are more susceptible to acquiring the disease. Smallpox may remodel bone in the form of osteomyelitis variolosa (‘smallpox arthritis’) (Aufderheide & Rodríguez-Martín 1998; Jackes 1983; Ortner & Putschar 1985) which causes the reduction of longitudinal bone growth (Jackes 1983). However, since smallpox only remodels bone in very few individuals and solely in children the only method for unconditionally determining the presence of the smallpox virus in a skeletal population is by performing DNA and PCR analyses. Survival from smallpox affords the individual natural immunity for the remainder of their life. The virus is undetectable in a smallpox survivor as they will possess the antibodies for the disease and therefore will have gained natural immunity for the remainder of his or her life. -

Thesis Hum 2007 Mcdonald J.Pdf
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town 'When Shall These Dry Bones Live?' Interactions between the London Missionary Society and the San Along the Cape's North-Eastern Frontier, 1790-1833. Jared McDonald MCDJAR001 A dissertation submitted in fulfillment of the requirements for the award of the degree of Master of Arts in Historical Studies Faculty of the Humanities University of Cape Town 2007 University of Cape Town COMPULSORY DECLARATION This work has not been previously submitted in whole, or in part, for the award of any degree. It is my own work. Each significant contribution to, and quotation in, this dissertation from the work, or works, of other people has been attributed, and has been cited and referenced. Signature:_fu""""',_..,"<_l_'"'"-_(......\b __ ~_L ___ _ Date: 7 September 2007 "And the Lord said to me, Prophe,\y to these bones, and scry to them, o dry bones. hear the Word ofthe Lord!" Ezekiel 37:4 Town "The Bushmen have remained in greater numbers at this station ... They attend regularly to hear the Word of God but as yet none have experiencedCape the saving effects ofthe Gospe/. When shall these dry bones oflive? Lord thou knowest. -

Xhariep Magisterial District
!. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. !. XXhhaarriieepp MMaaggiisstteerriiaall DDiissttrriicctt !. !. !. !. !. !. !. !. !. !. TheunissenS ubD istrict !. BARKLY WEST R59 R707 !. ST DEALESVILLE R708 SAPS WINBURG ST R70 !. R370 Lejwelepuitsa SAPS Dealesville R73 Winburg ST ST ST R31 Lejwelepuitsa ST Marquard !. ST LKN12 Boshof !. BRANDFORT Brandfort SAPS !. Soutpan R703 SAPS !. R64 STR64 Magiisteriiall R703 !. ST Sub District Marquard Senekal CAMPBELL ST Kimberley Dealesville ST Ficksburg !. !. !. !. R64 Sub BOSHOF SOUTPAN SAPS KIMBERLEY ST Sub !. !. !. SAPS Diistriict R64 SAPS Brandfort !. SAPS ST R709 Sub District District Sub !. !. Sub ST District Verkeerdevlei MARQUARD Sub District N1 !. SAPS Clocolan !. !. District STR700 KL District -
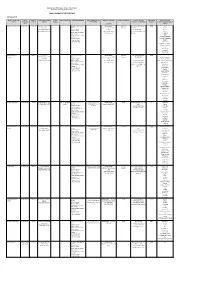
Public Libraries in the Free State
Department of Sport, Arts, Culture & Recreation Directorate Library and Archive Services PUBLIC LIBRARIES IN THE FREE STATE MOTHEO DISTRICT NAME OF FRONTLINE TYPE OF LEVEL OF TOWN/STREET/STREET STAND GPS COORDINATES SERVICES RENDERED SPECIAL SERVICES AND SERVICE STANDARDS POPULATION SERVED CONTACT DETAILS REGISTERED PERIODICALS AND OFFICE FRONTLINE SERVICE NUMBER NUMBER PROGRAMMES CENTER/OFFICE MANAGER MEMBERS NEWSPAPERS AVAILABLE IN OFFICE LIBRARY: (CHARTER) Bainsvlei Public Library Public Library Library Boerneef Street, P O Information and Reference Library hours: 446 142 Ms K Niewoudt Tel: (051) 5525 Car SA Box 37352, Services Ma-Tue, Thu-Fri: 10:00- (Metro) 446-3180 Fair Lady LANGENHOVENPARK, Outreach Services 17:00 Fax: (051) 446-1997 Finesse BLOEMFONTEIN, 9330 Electronic Books Wed: 10:00-18:00 karien.nieuwoudt@mangau Hoezit Government Info Services Sat: 8:30-12:00 ng.co.za Huisgenoot Study Facilities Prescribed books of tertiary Idees Institutions Landbouweekblad Computer Services: National Geographic Internet Access Rapport Word Processing Rooi Rose SA Garden and Home SA Sports Illustrated Sarie The New Age Volksblad Your Family Bloemfontein City Public Library Library c/o 64 Charles Information and Reference Library hours: 443 142 Ms Mpumie Mnyanda 6489 Library Street/West Burger St, P Services Ma-Tue, Thu-Fri: 10:00- (Metro) 051 405 8583 Africa Geographic O Box 1029, Outreach Services 17:00 Architect and Builder BLOEMFONTEIN, 9300 Electronic Books Wed: 10:00-18:00 Tel: (051) 405-8583 Better Homes and Garden n Government Info -

Review of Existing Infrastructure in the Orange River Catchment
Study Name: Orange River Integrated Water Resources Management Plan Report Title: Review of Existing Infrastructure in the Orange River Catchment Submitted By: WRP Consulting Engineers, Jeffares and Green, Sechaba Consulting, WCE Pty Ltd, Water Surveys Botswana (Pty) Ltd Authors: A Jeleni, H Mare Date of Issue: November 2007 Distribution: Botswana: DWA: 2 copies (Katai, Setloboko) Lesotho: Commissioner of Water: 2 copies (Ramosoeu, Nthathakane) Namibia: MAWRD: 2 copies (Amakali) South Africa: DWAF: 2 copies (Pyke, van Niekerk) GTZ: 2 copies (Vogel, Mpho) Reports: Review of Existing Infrastructure in the Orange River Catchment Review of Surface Hydrology in the Orange River Catchment Flood Management Evaluation of the Orange River Review of Groundwater Resources in the Orange River Catchment Environmental Considerations Pertaining to the Orange River Summary of Water Requirements from the Orange River Water Quality in the Orange River Demographic and Economic Activity in the four Orange Basin States Current Analytical Methods and Technical Capacity of the four Orange Basin States Institutional Structures in the four Orange Basin States Legislation and Legal Issues Surrounding the Orange River Catchment Summary Report TABLE OF CONTENTS 1 INTRODUCTION ..................................................................................................................... 6 1.1 General ......................................................................................................................... 6 1.2 Objective of the study ................................................................................................ -

South African Jewish Board of Deputies Report of The
The South African Jewish Board of Deputies JL 1r REPORT of the Executive Council for the period July 1st, 1933, to April 30th, 1935. To be submitted to the Eleventh Congress at Johannesburg, May 19th and 20th, 1935. <י .H.W.V. 8. Co י É> S . 0 5 Americanist Commiitae LIBRARY 1 South African Jewish Board of Deputies. EXECUTIVE COUNCIL. President : Hirsch Hillman, Johannesburg. Vice-President» : S. Raphaely, Johannesburg. Morris Alexander, K.C., M.P., Cape Town. H. Moss-Morris, Durban. J. Philips, Bloemfontein. Hon. Treasurer: Dr. Max Greenberg. Members of Executive Council: B. Alexander. J. Alexander. J. H. Barnett. Harry Carter, M.P.C. Prof. Dr. S. Herbert Frankel. G. A. Friendly. Dr. H. Gluckman. J. Jackson. H. Katzenellenbogen. The Chief Rabbi, Prof. Dr. j. L. Landau, M.A., Ph.D. ^ C. Lyons. ^ H. H. Morris Esq., K.C. ^י. .B. L. Pencharz A. Schauder. ^ Dr. E. B. Woolff, M.P.C. V 2 CONSTITUENT BODIES. The Board's Constituent Bodies are as. follows :— JOHANNESBURG (Transvaal). 1. Anykster Sick Benefit and Benevolent Society. 2. Agoodas Achim Society. 3. Beth Hamedrash Hagodel. 4. Berea Hebrew Congregation. 5. Bertrams Hebrew Congregation. 6. Braamfontein Hebrew Congregation. 7. Chassidim Congregation. 8. Club of Polish Jews. 9. Doornfontein Hebrew:: Congregation.^;7 10. Eastern Hebrew Benevolent Society. 11. Fordsburg Hebrew Congregation. 12. Grodno Sifck Benefit and Benevolent Society. 13. Habonim. 14. Hatechiya Organisation. 15. H.O.D. Dr. Herzl Lodge. 16. H.O.D. Sir Moses Montefiore Lodge. 17. Jeppes Hebrew Congregation, 18. Johannesburg Jewish Guild. 19. Johannesburg Jewish Helping Hand and Burial Society. -

Agri-Park District: Xhariep Province: Free State Reporting Date: March 2016 Key Commodities Agripark Components Status
AGRI-PARK DISTRICT: XHARIEP PROVINCE: FREE STATE REPORTING DATE: MARCH 2016 KEY COMMODITIES AGRIPARK COMPONENTS STATUS 13 FPSUs to be located in: Petrusburg, Edenburg, Red meat (beef and mutton) Luckoff, Smithfield, Bethulie, Koffiefontein, Fauresmith, DAMC Established Venison Zastron, Jacobsdal, Springfontein, Reddersburg, 16 members Aquaculture Philippolis, Rouxville The final Master Business plan was submitted on 29/02/2016 1 Agri-Hub located in Springfontein KEY CATALYTIC PROJECTS 1 AGRORUMC -locatedPROCESSING in Bloemfontein BUSINESS OPPORTUNITIES KEY ROLE-PLAYERS Building of an export abattoir that will have Beef: abattoir; freezing; primary butchering; grading Public Sector Industry Other four processing lines for venison, cattle, and labelling; transporting; washing; cleaning; DRDLR SA Veterinary Association ARC sheep and potentially ostrich. The abattoir packaging; storage; leather tanning; semi-dry, DRDAR Red Meat Producers Organisation NEF will be responsible for large scale Dept. of Kroonstad Piggery Farmers MAFISA slaughtering, processing, deboning and cooked and smoked sausages, Agriculture NERPO Glen packaging of different types of meat as Mutton: packaging and branding, drying of pastirma (Petrusburg/ IMQAS Agriculture while adhering to international export and mutton jerky and offal marketing Zastron/ Sparta Foods Institute Koffiefontein) SAMIC SEDA standards. Venison: Mobile abattoirs; game fencing; organic FS Dept. of Association of Meat Importers and Exporters DBSA Development or refurbishment -

A Regional Analysis of Agricultural Price Risk in South Africa
Agrekon, Vol 33, No 3 (September 1994) Van Schalkwyk and Groenewald A REGIONAL ANALYSIS OF AGRICULTURAL PRICE RISK IN SOUTH AFRICA HD van Schalkwyk and JA Groenewald Lecturer and Professor, Department ofAgricultural Economics, Extension and Rural Development, University ofPretoria, Pretoria Regional output/input price differentials and variations were calculated to evaluate price risk in South Africa. It was found that price unstable regions are not necessarily also risky regions as regions with higher output/input price ratios can handle higher price variations better. The average value of the price index for the top ten regions is over 3 times larger than the average for the ten lowest regions. The higher the prices of inputs relative to output prices (the lower the price ratio), the smaller their application to each hectare of land, and the lower the land productivity. The regional prices appears to be a function of the interaction between differential natural and economic factors in different regions. 'n Streeksanalise van landbou prysrisiko in Suid-Afrika Regionale uitset/inset prysverskille en variasies is bereken om prys risiko in Suid-Afrika te evalueer. Daar is gevind dat prys onstabiele streke the noodwendig ook hoe risiko streke is nie, want streke met hoer uitset/inset prys verhoudings kan hoer prys variasies beter verwerk. Die gemiddelde waarde van die prys indeks vir die tien top streke is nicer as 3 keer groter as die gemiddeld vir die tien laagste streke. Hoe hoer die pryse van insette relatief tot uiset pryse(hoe lac- die prys verhouding), hoe minder is hulle aanwending per hektaar, en hoe laer die grond produktiwiteit. -

Free State Province
Agri-Hubs Identified by the Province FREE STATE PROVINCE 27 PRIORITY DISTRICTS PROVINCE DISTRICT MUNICIPALITY PROPOSED AGRI-HUB Free State Xhariep Springfontein 17 Districts PROVINCE DISTRICT MUNICIPALITY PROPOSED AGRI-HUB Free State Thabo Mofutsanyane Tshiame (Harrismith) Lejweleputswa Wesselsbron Fezile Dabi Parys Mangaung Thaba Nchu 1 SECTION 1: 27 PRIORITY DISTRICTS FREE STATE PROVINCE Xhariep District Municipality Proposed Agri-Hub: Springfontein District Context Demographics The XDM covers the largest area in the FSP, yet has the lowest Xhariep has an estimated population of approximately 146 259 people. population, making it the least densely populated district in the Its population size has grown with a lesser average of 2.21% per province. It borders Motheo District Municipality (Mangaung and annum since 1996, compared to that of province (2.6%). The district Naledi Local Municipalities) and Lejweleputswa District Municipality has a fairly even population distribution with most people (41%) (Tokologo) to the north, Letsotho to the east and the Eastern Cape residing in Kopanong whilst Letsemeng and Mohokare accommodate and Northern Cape to the south and west respectively. The DM only 32% and 27% of the total population, respectively. The majority comprises three LMs: Letsemeng, Kopanong and Mohokare. Total of people living in Xhariep (almost 69%) are young and not many Area: 37 674km². Xhariep District Municipality is a Category C changes have been experienced in the age distribution of the region municipality situated in the southern part of the Free State. It is since 1996. Only 5% of the total population is elderly people. The currently made up of four local municipalities: Letsemeng, Kopanong, gender composition has also shown very little change since 1996, with Mohokare and Naledi, which include 21 towns. -

Pest-Free Areas
Pest-Free Areas The following table lists countries and associated areas that meet the APHIS requirements for designated pest-free areas in accordance with 7 CFR 319.56- 5. Country Pest(s) Pest-free Area Argentina Mediterranean fruit fly (Medfly) Cer- The Patagonia Provinces of Neuguen, Rio Negro, atitis capitata and Anastrepha spp. Chabut, Santa Cruz, and Tierra del Fuego. This includes fruit flies areas along the valleys of the Rio Colorado and Rio Negro rivers and areas of the southern part of the Men- doza Province, south of the following coordinates: lat 33o 13’ 40.98” S, length 69o 54’ 36.86” W; lat 33o 13’ 40.98” S, length 69o 04’ 18.24” W; lat 33o 29’ 29” S, length 68o 59’ 20” W; lat 34o 02’ 47” S, length 67o 57’ 17” W; lat 34o 02’ 47” S, length 66o 44’ 06.05” W Australia Mediterranean fruit fly (Medfly) (Cer- The Riverland district of South Australia, defined as the atitis capitata), the Queensland fruit county of Hamley; the geographical subdivisions, called fly (Bactrocera tryoni or QFF) and hundreds, of Bookpurnong, Cadell, Eba, Fisher, Forster, other fruit flies destructive of citrus Gordon, Hay, Holder, Katarapko, Loveday, Markaranka, Morook, Murbko, Murtho, Nildottie, Paisley, Parcoola, Paringa, Pooginook, Pyap, Ridley, Skurray, Stuart, and Waikerie; and the Parish of Onley of the Shire of Mildura, Victoria Mediterranean fruit fly (Medfly) (Cer- Tasmania atitis capitata) and the Queensland fruit fly (Bactrocera tryoni) Mediterranean fruit fly (Medfly) (Cer- Eastern Australia, defined as the Northern Territory, atitis capitata) -
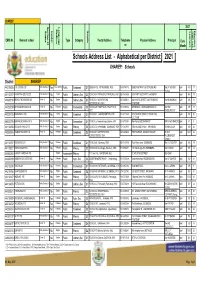
Xhariep Address List 3 May 2021.Pdf
XHARIEP Section 21 Language 2021 Medium Quintile Educators Educators Hostel Status Learners (SGB incl) (SGB EMIS Nr. Name of school Type Category Postal Address Telephone Physical Address Principal Data nr. Month Schools Address List - Alphabetical per District 2021 XHARIEP: Schools District: XHARIEP 443104215 AJC JOOSTE C/S Partly Section 21 Yes Dual: Afr/Eng Public Combined Q1 OSSEWA 38, , PETRUSBURG, 9932 053-5740176 OSSEWASTRAAT 38, PETRUSBURG Mej CF VENTER April 410 17 (Acting) 441103107 ALBERTINA SISULU S/S Partly Section 21 No English Public Ordinary Sec. Q2 PO BOX 58, EDENBURG, EDENBURG, 9908 051-7431859 1679 PHETLHU STREET, HARASEBEI April 562 19 443303110 BEANG TSE MOLEMO S/S Section 21 No English Public Ordinary Sec. Q3 PO BOX 21, MATOPORONG, 067-2250611 832 KHUTSO STREET, MATOPORONG Mrs MB MASHOAI April 418 14 REDDERSBURG, 9904 TOWNSHIP 443203219 BERGMANSHOOGTE I/S Section 21 No Afrikaans Public Intermediate Q3 PO BOX 29, PHILIPPOLIS, PHILIPPOLIS, 051-7730104 ARENDWEG 1, BERGMANSHOOGTE Mnr RMT April 419 17 9970 ENGELBRECHT 442204126 BOARAMELO C/S Partly Section 21 No English Public Combined Q1 PO BOX 31, , JAGERSFONTEIN, 9974 081-3111033 1295 SEEKOEI STREET, ITUMELENG April 914 30 LOCATION 445203174 BOKAMOSO/IKAMVA IF/S Partly Section 21 No English Farm Intermediate Q1 PO BOX 22, Boesmanksop, Zastron, 9951 051-6731038 Main Road, BOESMANSKOP MR RJ MOFAMMERE April 97 5 441103256 BOTLE BA THUTO P/S Partly Section 21 No English Public Primary Q2 PO BOX 54, HA-RASEBEI, EDENBURG, 9908 051-7431803 1044 VELEKO STREET, HARASEBEI Mr MW LEEUW April 820 24 442304245 DIAMANTHOOGTE C/S Section 21 Yes Afrikaans Public Combined Q2 PO BOX 97, KOFFIEFONTEIN, 067-2245606 FIRST AVENUE, DIAMANTHOOGTE Mr.