Abernathys Surgical Secrets 6Th Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

446 NQF #0773: Operative Mortality Stratified by the Five STS-EACTS Mortality Categories
Measure #446 (NQF 0733): Operative Mortality Stratified by the Five STS-EACTS Mortality Categories – National Quality Strategy Domain: Patient Safety 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION: Percent of patients undergoing index pediatric and/or congenital heart surgery who die, including both 1) all deaths occurring during the hospitalization in which the procedure was performed, even if after 30 days (including patients transferred to other acute care facilities), and 2) those deaths occurring after discharge from the hospital, but within 30 days of the procedure, stratified by the five STAT Mortality Levels, a multi-institutional validated complexity stratification tool INSTRUCTIONS: This measure is to be reported for all pediatric and/or congenital heart patients each time a surgery is performed during the performance period. This measure is intended to reflect the quality of services provided for patients with congenital heart disease. This measure may be reported by eligible clinicians who perform the quality actions described in the measure based on the services provided and the measure-specific denominator coding. Measure Reporting: The listed denominator criteria is used to identify the intended patient population. The numerator quality-data codes included in this specification are used to submit the quality actions allowed by the measure. All measure-specific coding should be reported on the claim(s) representing the eligible encounter. THERE ARE TWO REPORTING CRITERIA FOR THIS MEASURE: -

Situs Inversus Totalis with Aortopulmonary Shunt: a Case Report in an Ethiopian
Ethiop. J. Health Biomed Sci., 2009. Vol.2, No.1 CASE REPORT SITUS INVERSUS TOTALIS WITH AORTOPULMONARY SHUNT: A CASE REPORT IN AN ETHIOPIAN Ermias Diro1 SUMMARY I report a patient who presented with long standing dyspnea without physical signs of congestive heart failure and finally diag- nosed to have situs inversus totalis with aortopulmonary window. Situs inversus totalis refers to a mirror image reversal of the normal position of the internal organs. The recognition of concomitant congenital anomalies such as in the heart or other or- gans is extremely important as it may disturb surgical procedures for concomitant diseases. This is a very rare condition and a coexisting aortopulmonary window was not described before to the best of my knowledge. their normal positions. Situs inversus with dextrocar- dia is termed situs inversus totalis because the car- INTRODUCTION diac position as well as the atrial chambers and ab- dominal viscera are mirror images of the normal anatomy (3). The purpose of this case report is to describe and discuss a rare situation with an important clinical Typically, patients with situs inversus have a normal significance. life expectancy except in the rare instances of cardiac anomalies, depending on the severity of the defect. Situs describes the position of the cardiac atria and Patients with Kartagners syndrome, a triad of situs viscera. Situs solitus is the normal position, and situs inversus, sinusitis and bronchiectasis, have a normal inversus is the mirror image of situs solitus. Cardiac life expectancy if the bronchiectasis is treated ade- situs is determined by the atrial location. In situs in- quately. -

Game Summary
On Pettlll's Double In 7th- Tigers Oust Breda In Sectional Fray, 4-3 Bruce Pettitt rifled, a run- den-Ralston, 10-0, in the first Peterson and Brechler were and Jim Kennebeck to place three down in the bottom of the Timet Herald, Carroll, Iowa C apiece. Polking, R., rf .. 2 0 0 0 scoring clutch shot to deep right also the big men in the hitting the Bobcats in scoring position. sixth frame before the Bobcats round, against the Lake City Thursday, July 16, 1970 ^ Nieland, 2b 2 10 0 field in the bottom of the line-up for Carroll. Peterson Bob Polking fanned for theforce d a deadlock in the top The Carroll club took ad Eagles. That game is sched Buelt, lb 2 10 0 seventh frame to give the Car stroked a pair of singles and first out, but Jerry Roster's of the seventh. vantage of their last chance Peterson followed with a one- to break the tie. Anderson Totals 21 3 2 1 roll Tigers a 4-3 edge over the uled for 6 p.m. a booming triple to right field, slow grounder between short bagger to score Brechler for Russ Polking took a base on walked and stole second base Breda Bobcats of St. Bernard's while Brechler cracked a one- stop and third base was left CARROLL the second tally. Roger Fuller balls in the lead off role in the to give the Tigers a scoring High School in the opening AB K H Bf Carroll hurlers, Doug Peter bagger and an inside-the-park flew out to center field to re untouched to load the sacks. -

My Replay Baseball Encyclopedia Fifth Edition- May 2014
My Replay Baseball Encyclopedia Fifth Edition- May 2014 A complete record of my full-season Replays of the 1908, 1952, 1956, 1960, 1966, 1967, 1975, and 1978 Major League seasons as well as the 1923 Negro National League season. This encyclopedia includes the following sections: • A list of no-hitters • A season-by season recap in the format of the Neft and Cohen Sports Encyclopedia- Baseball • Top ten single season performances in batting and pitching categories • Career top ten performances in batting and pitching categories • Complete career records for all batters • Complete career records for all pitchers Table of Contents Page 3 Introduction 4 No-hitter List 5 Neft and Cohen Sports Encyclopedia Baseball style season recaps 91 Single season record batting and pitching top tens 93 Career batting and pitching top tens 95 Batter Register 277 Pitcher Register Introduction My baseball board gaming history is a fairly typical one. I lusted after the various sports games advertised in the magazines until my mom finally relented and bought Strat-O-Matic Football for me in 1972. I got SOM’s baseball game a year later and I was hooked. I would get the new card set each year and attempt to play the in-progress season by moving the traded players around and turning ‘nameless player cards” into that year’s key rookies. I switched to APBA in the late ‘70’s because they started releasing some complete old season sets and the idea of playing with those really caught my fancy. Between then and the mid-nineties, I collected a lot of card sets. -

All Forms Combined
Not Started Institutional Practice Details Print this Form Date of Completion DD/MM/YYYY 1. Indicate the day, month, and year the form is being completed. Previous year’s hospital case Less than or equal to 100 per year 2. volume of congenital cardiac 101-250 per year surgeries 251-500 per year Indicate the case volume of Tier 1 AND Tier 2 surgeries for the previous Greater than 500 per year calendar year. (This is the total number of surgeries, not the number of patients.) Active congenital heart surgeons active congenital heart 3. Indicate the number of active congenital heart surgeons currently surgeons practicing at your hospital. How is a congenital heart 3a surgeon certified in your country? Cardioplegia Type Buckberg 4. Check all cardioplegia types that your hospital uses. If there are Custodiol/Bretschneider (HTK) multiple cardioplegia types that your hospital uses that are not Del Nido options in the list provided, enter all of them in the "other, specify" box Microplegia with Adenocaine seperating them by commas (,). 0 option(s) selected Microplegia with Potassium Plegisol/St. Thomas Roe's Solution University of Wisconsin Other, specify Geographic Region Served Local: one city or metro area 5. Regional: geographically larger than a metro area National: one country International: multiple countries Estimated Population Served Less than 10 million 6. Based on answer to the previous question. 10-30 million 31-50 million Greater than 51 million Unknown Total number of institutions Missing Reason: 7. providing pediatric cardiac Clear Unknown services in the region. Based on answer to question 5. Specify the total number including your institution. -

Klinička Slika Kasne Novorođenačke Sepse – I Dalje Ozbiljan Diferencijalnodijagnostički Problem
Liječ Vjesn 2019;141:150–161 https://doi.org/10.26800/LV-141-5-6-21 Pregled | Review Klinička slika kasne novorođenačke sepse – i dalje ozbiljan diferencijalnodijagnostički problem Clinical manifestation of late neonatal sepsis – continuous differential diagnostic dilemma Matej Katavić1, Andrea Dasović Buljević2 1 Klinika za pedijatriju, KBC Sestre milosrdnice 2 Zavod za neonatologiju i neonatalno intenzivno liječenje, Klinika za pedijatriju, Medicinski fakultet Sveučilišta u Zagrebu, KBC Zagreb Deskriptori SAŽETAK. Novorođenačka sepsa kao klinički sindrom jedan je od najčešćih dijagnostičko-terapijskih izazova u NOVOROĐENAČKA SEPSA – dijagnoza, etiologija, novorođenačkoj dobi s relativno visokom stopom smrtnosti. Prema dobi manifestacije, dijeli se na ranu i kasnu liječenje; SEPTIČKI ŠOK – dijagnoza, liječenje; sepsu, ovisno o izvorima i mišljenjima raznih stručnjaka. Može se očitovati poremećajem svih organskih sustava i DIFERENCIJALNA DIJAGNOZA; brojnim kasnim komplikacijama te znatnim morbiditetom i mortalitetom. Razdoblje od drugog tjedna života NOVOROĐENAČKE BOLESTI – dijagnoza, liječenje primarni je interes ovog rada jer je u tom periodu (dotad prividno zdrava) novorođenčad otpuštena kući iz rodi- lišta, adaptirana na svakodnevne zahtjeve ekstrauterinog života, s mogućnošću razvoja niza neinfektivnih stanja i bolesti koji klinički izgledaju kao septičko stanje. Široka diferencijalna dijagnoza takvog stanja može biti ozbiljan problem koji nalaže hitnu reakciju, pravilno i pravodobno liječenje te, naposljetku, zbrinjavanje u adekvatnoj -

Cardiovascular Magnetic Resonance (CMR) Page 1 of 11
Cardiovascular Magnetic Resonance (CMR) Page 1 of 11 No review or update is scheduled on this Medical Policy as it is unlikely that further published literature would change the policy position. If there are questions about coverage of this service, please contact Blue Cross and Blue Shield of Kansas customer service, your professional or institutional relations representative, or submit a predetermination request. Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cardiovascular Magnetic Resonance (CMR) Professional Institutional Original Effective Date: August 4, 2005 Original Effective Date: July 1, 2006 Revision Date(s): February 27, 2006, Revision Date(s): May 2, 2007; May 2, 2007; November 1, 2007; November 1, 2007; January 1, 2010; January 1, 2010; February 15, 2013; February 15, 2013; December 11, 2013; December 11, 2013; April 15, 2014; April 15, 2014; July 15, 2014; June 10, 2015; July 15, 2014; June 10, 2015; June 8, 2016; June 8, 2016; October 1, 2016; October 1, 2016; May 10, 2017; May 10, 2017; April 25, 2018; April 25, 2018; October 1, 2018 October 1, 2018 Current Effective Date: July 15, 2014 Current Effective Date: July 15, 2014 Archived Date: July 3, 2019 Archived Date: July 3, 2019 State and Federal mandates and health plan member contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. To verify a member's benefits, contact Blue Cross and Blue Shield of Kansas Customer Service. The BCBSKS Medical Policies contained herein are for informational purposes and apply only to members who have health insurance through BCBSKS or who are covered by a self-insured group plan administered by BCBSKS. -

Unifocalization of Major Aortopulmonary Collaterals in Single-Ventricle Patients Olaf Reinhartz, MD, V
CARDIOVASCULAR Unifocalization of Major Aortopulmonary Collaterals in Single-Ventricle Patients Olaf Reinhartz, MD, V. Mohan Reddy, MD, Edwin Petrossian, MD, Sam Suleman, BS, Richard D. Mainwaring, MD, David N. Rosenthal, MD, Jeffrey A. Feinstein, MD, Raj Gulati, MD, and Frank L. Hanley, MD Department of Cardiothoracic Surgery, Division of Pediatric Cardiac Surgery, and Department of Pediatrics, Division of Pediatric Cardiology, Stanford University, Stanford, California Background. Unifocalization of major aortopulmonary procedures. Median postoperative pulmonary artery collateral arteries (MAPCAs) in pulmonary atresia with pressures measured 12.5 mm Hg (Glenn) and 14 mm Hg ventricular septal defect and intracardiac repair has be- (Fontan), respectively. Six patients are alive today (46%), come the standard of care. However, there are no reports with 1 patient lost to follow-up. Three patients died early addressing unifocalization of MAPCAs in single-ventri- and 3 late after initial unifocalization to shunts. One cle patients. It is unknown whether their pulmonary other patient survived unifocalization, but was not con- vascular bed can be reconstructed and low enough pul- sidered a candidate for a Glenn procedure and died after monary vascular resistance achieved to allow for superior high-risk two-ventricle repair. Another patient with or total cavopulmonary connections. right-ventricle–dependent coronary circulation died of Methods. We reviewed data on all patients with func- sepsis late after Glenn. tional single ventricles and unifocalization procedures of Conclusions. In selected patients with functional single MAPCAs. From 1997 to 2005, 14 consecutive children ventricles and MAPCAs, the pulmonary vascular bed can with various single-ventricle anatomies were operated be reconstructed sufficiently to allow for cavopulmonary on. -

Probable Starting Pitchers 29-30, Home 13-15, Road 16-15
NOTES Great American Ball Park • 100 Joe Nuxhall Way • Cincinnati, OH 45202 • @Reds • @RedsPR • @RedlegsJapan • reds.com 29-30, HOME 13-15, ROAD 16-15 PROBABLE STARTING PITCHERS Thursday, June 10, 2021 Thu vs Mil: RHP Luis Castillo (2-8, 6.63) vs RHP Freddy Peralta (6-1, 2.25) 700 wlw, bsoh, 12:35et Fri vs Col: RHP Tyler Mahle (5-2, 3.32) vs LHP Kyle Freeland (0-1, 6.23) 700 wlw, bsoh, 7:10et Great American Ball Park Sat vs Col: LHP Wade Miley (5-4, 2.96) vs RHP Germán Márquez (4-5, 3.91) 700 wlw, bsoh, 4:10et • • • • • • • • • • Sun vs Col: TBD vs RHP Antonio Senzatela (2-6, 4.62) 700 wlw, bsoh, 1:10et Mon at Mil: RHP Vladimir Gutierrez (2-1, 2.65) vs TBD 700 wlw, bsoh, 8:10et CINCINNATI REDS (29-30) vs Tue at Mil: RHP Luis Castillo vs TBD 700 wlw, bsoh, 8:10et MILWAUKEE BREWERS (34-27) Wed at Mil: RHP Tyler Mahle vs TBD 700 wlw, bsoh, mlbn backup, 2:10et TODAY'S GAME: Is Game 3 (1-1) of a 3-game series vs Andrew Gruman's STREAKING REGULAR SEASON RECORD VS BREWERS second-place Brewers and Game 3 (1-1) of a 6-game homestand that The Reds have of their All-Time Since 1997: ............................. * 196-190-1 won 7 last 9 games includes an upcoming 3-game weekend series vs the Rockies...the Reds since , when they were At Cinergy Field: ........................................... 20-20-1 5/29 6 games under are coming off a brief 4-game road trip, their first 4-game series sweep (22-28) and out of first place, At Great American Ball Park: ................. -

No-Hitter Doesn't Count
D^TRIBUME-EXAMINER DUlon, Montan» ' Page 6 Thursday, August,1974 22 Coach different, result sai> 1* for last-place Jacksonville By BRUCE LOWITT showed they still have the knack.Portland Storm 11-7. The Chicago AP Sports Writer Reed, Birmingham’s rookie Fire visits the Detroit Wheels Welcome to the World Football quarterback from Gramblingtonight. League, Charlie Tate. who has his own knack of taking Grant Guthrie had kicked field And welcome back, Matthew over in style when veterangoals of 31 and 51 yards to give Reed. George Mira gets hurt, did it the Sharks a 6-0 lead going into Tate coached his first game again. He drove the Americans tothe final period. But Reed threw a Wednesday night—and musttwo touchdowns in the fourth27-yard touchdown pass to Alfred knbw exactly how Bud Asher felt.quarter—the second one comingJenkins to put the Americans Asha* was fired by Jackson with barely a minute to play— forahead. ville last Saturday after thea 15-14 victory over the Sharks. Thai, after the Jacksonville Sharks, who had a knack of losing In Wednesday night’s othertouchdown moved the Sharks close games in the closing WFL games, the New York Stars back in front, Reed mounted the minutes, had dropped to a 2-4 demolished the Houston Texanswinning touchdown drive. mark. 43-10, the Memphis Southmen Stars 43, Texans 10 Tate's debut came against obliterated the Hawaiians 60-8, New York’s Bob Gladieux unbeaten Birmingham. the Southern California Sunmade it look easy. He ran for 84 The result: The Americans bested the Philadelphia Bell 31-18 yards and three touchdowns and stayed unbeaten and the Sharksand the Florida Blazers beat the caught a Tom Sherman pass for a fourth score in the Stars’ rout of Houston for their fifth straight victory. -
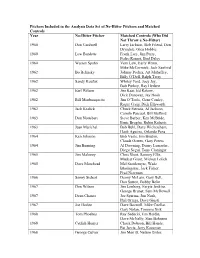
Pitchers Included in the Analysis Data Set of No-Hitter Pitchers and Matched Controls
Pitchers Included in the Analysis Data Set of No-Hitter Pitchers and Matched Controls Year No-Hitter Pitcher Matched Controls (Who Did Not Throw a No-Hitter) 1960 Don Cardwell Larry Jackson, Bob Friend, Don Drysdale, Glen Hobbie 1960 Lew Burdette Frank Lary, Jim Perry, Pedro Ramos, Bud Daley 1960 Warren Spahn Vern Law, Early Wynn, Mike McCormick, Jack Sanford 1962 Bo Belinsky Johnny Podres, Art Mahaffey, Billy O’Dell, Ralph Terry 1962 Sandy Koufax Whitey Ford, Joey Jay, Bob Purkey, Ray Herbert 1962 Earl Wilson Jim Kaat, Ed Rakow, Dick Donovan, Jay Hook 1962 Bill Monbouquette Jim O’Toole, Gene Conley, Roger Craig, Dick Ellsworth 1962 Jack Kralick Chuck Estrada, Al Jackson, Camilo Pascual, Bill Stafford 1963 Don Nottebart Steve Barber, Ken McBride, Ernie Broglio, Robin Roberts 1963 Juan Marichal Bob Buhl, Dave Wickersham, Hank Aguirre, Orlando Pena 1964 Ken Johnson Bob Veale, Jim Bouton, Claude Osteen, Gary Peters 1964 Jim Bunning Al Downing, Denny Lemaster, Diego Segui, Tony Cloninger 1965 Jim Maloney Chris Short, Sammy Ellis, Mudcat Grant, Mickey Lolich 1965 Dave Morehead Mel Stottlemyre, Wade Blasingame, Jack Fisher, Fred Newman 1966 Sonny Siebert Denny McLain, Gary Bell, Don Sutton, Bobby Bolin 1967 Don Wilson Jim Lonborg, Fergie Jenkins, George Brunet, Sam McDowell 1967 Dean Chance Joe Sparma, Jim Nash, Phil Ortega, Dave Giusti 1967 Joe Horlen Dave Boswell, Mike Cuellar, Gary Nolan, Tommie Sisk 1968 Tom Phoebus Ray Sadecki, Jim Hardin, Dave McNally, Stan Bahnsen 1968 Catfish Hunter Chuck Dobson, Bill Hands, Pat Jarvis, Jerry Koosman -

We Are Turning 40!
Page 1 KIT YOUNG’S SALE #128 WE ARE TURNING 40! This month marks our 40th year in business. I started back in the stone ages as a one-man gang, mainly doing shows and eventually advertising in The Trader Speaks, Sports Collectors Digest, then later in Baseball Hobby News, The Sporting News, USA Weekly, The Advertiser and more. I was joined by Scott Cowan when he was a mere child of 15 (he’s now 51), then within a short time by Rob Rosen, Bob Ivanjack & Nacho Arredondo. These 4 stalwarts have been my partners for a combined 110 years (average 27 years each). I’ve noted before that we started before there were official price guides and long before words like “faxing”, “emailing” and “Googling” were part of our world. It’s hard to believe but we opened our doors before Microsoft, Home Depot & Costco even existed. It’s been a great ride so far. Some of our clients have dealt with us for 20-30 years, some longer. It’s still a kick to go to a big card show and have people stop by to say they ordered their first card from us. They usually say they ordered with they were 9-12. These collectors now are often 40-50. Crazy! Special thanks to the 100,000+ collectors we’ve served over the past 4 decades. I look forward to the coming years. Kit, our late leader Patti, Scott, Bob, Nacho & Rob To celebrate this anniversary we are offering special 20-30-40% off sections in this sale.