Evolutionary Ecology of Fish Venom: Adaptations and Consequences of Evolving a Venom System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Snake Bite Protocol
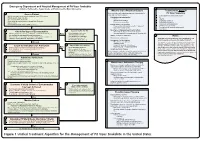
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

1 Exon Probe Sets and Bioinformatics Pipelines for All Levels of Fish Phylogenomics
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.18.949735; this version posted February 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Exon probe sets and bioinformatics pipelines for all levels of fish phylogenomics 2 3 Lily C. Hughes1,2,3,*, Guillermo Ortí1,3, Hadeel Saad1, Chenhong Li4, William T. White5, Carole 4 C. Baldwin3, Keith A. Crandall1,2, Dahiana Arcila3,6,7, and Ricardo Betancur-R.7 5 6 1 Department of Biological Sciences, George Washington University, Washington, D.C., U.S.A. 7 2 Computational Biology Institute, Milken Institute of Public Health, George Washington 8 University, Washington, D.C., U.S.A. 9 3 Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian 10 Institution, Washington, D.C., U.S.A. 11 4 College of Fisheries and Life Sciences, Shanghai Ocean University, Shanghai, China 12 5 CSIRO Australian National Fish Collection, National Research Collections of Australia, 13 Hobart, TAS, Australia 14 6 Sam Noble Oklahoma Museum of Natural History, Norman, O.K., U.S.A. 15 7 Department of Biology, University of Oklahoma, Norman, O.K., U.S.A. 16 17 *Corresponding author: Lily C. Hughes, [email protected]. 18 Current address: Department of Organismal Biology and Anatomy, University of Chicago, 19 Chicago, IL. 20 21 Keywords: Actinopterygii, Protein coding, Systematics, Phylogenetics, Evolution, Target 22 capture 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.18.949735; this version posted February 19, 2020. -

Traveler Information
Traveler Information QUICK LINKS Marine Hazards—TRAVELER INFORMATION • Introduction • Risk • Hazards of the Beach • Animals that Bite or Wound • Animals that Envenomate • Animals that are Poisonous to Eat • General Prevention Strategies Traveler Information MARINE HAZARDS INTRODUCTION Coastal waters around the world can be dangerous. Swimming, diving, snorkeling, wading, fishing, and beachcombing can pose hazards for the unwary marine visitor. The seas contain animals and plants that can bite, wound, or deliver venom or toxin with fangs, barbs, spines, or stinging cells. Injuries from stony coral and sea urchins and stings from jellyfish, fire coral, and sea anemones are common. Drowning can be caused by tides, strong currents, or rip tides; shark attacks; envenomation (e.g., box jellyfish, cone snails, blue-ringed octopus); or overconsumption of alcohol. Eating some types of potentially toxic fish and seafood may increase risk for seafood poisoning. RISK Risk depends on the type and location of activity, as well as the time of year, winds, currents, water temperature, and the prevalence of dangerous marine animals nearby. In general, tropical seas (especially the western Pacific Ocean) are more dangerous than temperate seas for the risk of injury and envenomation, which are common among seaside vacationers, snorkelers, swimmers, and scuba divers. Jellyfish stings are most common in warm oceans during the warmer months. The reef and the sandy sea bottom conceal many creatures with poisonous spines. The highly dangerous blue-ringed octopus and cone shells are found in rocky pools along the shore. Sea anemones and sea urchins are widely dispersed. Sea snakes are highly venomous but rarely bite. -

Amphibious Fishes: Terrestrial Locomotion, Performance, Orientation, and Behaviors from an Applied Perspective by Noah R
AMPHIBIOUS FISHES: TERRESTRIAL LOCOMOTION, PERFORMANCE, ORIENTATION, AND BEHAVIORS FROM AN APPLIED PERSPECTIVE BY NOAH R. BRESSMAN A Dissertation Submitted to the Graduate Faculty of WAKE FOREST UNIVESITY GRADUATE SCHOOL OF ARTS AND SCIENCES in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Biology May 2020 Winston-Salem, North Carolina Approved By: Miriam A. Ashley-Ross, Ph.D., Advisor Alice C. Gibb, Ph.D., Chair T. Michael Anderson, Ph.D. Bill Conner, Ph.D. Glen Mars, Ph.D. ACKNOWLEDGEMENTS I would like to thank my adviser Dr. Miriam Ashley-Ross for mentoring me and providing all of her support throughout my doctoral program. I would also like to thank the rest of my committee – Drs. T. Michael Anderson, Glen Marrs, Alice Gibb, and Bill Conner – for teaching me new skills and supporting me along the way. My dissertation research would not have been possible without the help of my collaborators, Drs. Jeff Hill, Joe Love, and Ben Perlman. Additionally, I am very appreciative of the many undergraduate and high school students who helped me collect and analyze data – Mark Simms, Tyler King, Caroline Horne, John Crumpler, John S. Gallen, Emily Lovern, Samir Lalani, Rob Sheppard, Cal Morrison, Imoh Udoh, Harrison McCamy, Laura Miron, and Amaya Pitts. I would like to thank my fellow graduate student labmates – Francesca Giammona, Dan O’Donnell, MC Regan, and Christine Vega – for their support and helping me flesh out ideas. I am appreciative of Dr. Ryan Earley, Dr. Bruce Turner, Allison Durland Donahou, Mary Groves, Tim Groves, Maryland Department of Natural Resources, UF Tropical Aquaculture Lab for providing fish, animal care, and lab space throughout my doctoral research. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Approach and Management of Spider Bites for the Primary Care Physician
Osteopathic Family Physician (2011) 3, 149-153 Approach and management of spider bites for the primary care physician John Ashurst, DO,a Joe Sexton, MD,a Matt Cook, DOb From the Departments of aEmergency Medicine and bToxicology, Lehigh Valley Health Network, Allentown, PA. KEYWORDS: Summary The class Arachnida of the phylum Arthropoda comprises an estimated 100,000 species Spider bite; worldwide. However, only a handful of these species can cause clinical effects in humans because many Black widow; are unable to penetrate the skin, whereas others only inject prey-specific venom. The bite from a widow Brown recluse spider will produce local symptoms that include muscle spasm and systemic symptoms that resemble acute abdomen. The bite from a brown recluse locally will resemble a target lesion but will develop into an ulcerative, necrotic lesion over time. Spider bites can be prevented by several simple measures including home cleanliness and wearing the proper attire while working outdoors. Although most spider bites cause only local tissue swelling, early species identification coupled with species-specific man- agement may decrease the rate of morbidity associated with bites. © 2011 Elsevier Inc. All rights reserved. More than 100,000 species of spiders are found world- homes and yards in the southwestern United States, and wide. Persons seeking medical attention as a result of spider related species occur in the temperate climate zones across bites is estimated at 50,000 patients per year.1,2 Although the globe.2,5 The second, Lactrodectus, or the common almost all species of spiders possess some level of venom, widow spider, are found in both temperate and tropical 2 the majority are considered harmless to humans. -

A Guide to Harmful and Toxic Creatures in the Goa of Jordan
Published by the Royal Marine Conservation Society of Jordan. P. O. Box 831051, Abdel Aziz El Thaalbi St., Shmesani 11183. Amman Copyright: © The Royal Marine Conservation Society of Jordan Reproduction of this publication for educational and other non- commercial purposes is authorized without prior written approval from the copyright holder provided the source is fully acknowledged. ISBN: 978-9957-8740-1-8 Deposit Number at the National Library: 2619/6/2016 Citation: Eid, E and Al Tawaha, M. (2016). A Guide to Harmful and Toxic Creature in the Gulf of Aqaba of Jordan. The Royal Marine Conservation Society of Jordan. ISBN: 978-9957-8740-1-8. Pp 84. Material was reviewed by Dr Nidal Al Oran, International Research Center for Water, Environment and Energy\ Al Balqa’ Applied University,and Dr. Omar Attum from Indiana University Southeast at the United State of America. Cover page: Vlad61; Shutterstock Library All photographs used in this publication remain the property of the original copyright holder, and it should not be reproduced or used in other contexts without permission. 1 Content Index of Creatures Described in this Guide ......................................................... 5 Preface ................................................................................................................ 6 Part One: Introduction ......................................................................................... 8 1.1 The Gulf of Aqaba; Jordan ......................................................................... 8 1.2 Aqaba; -

Coral Injuries Caused by Spirobranchus Opercula with and Without Epibiotic Turf Algae at Curaçao
Marine Biology (2019) 166:60 https://doi.org/10.1007/s00227-019-3504-6 SHORT NOTE Coral injuries caused by Spirobranchus opercula with and without epibiotic turf algae at Curaçao Bert W. Hoeksema1,2 · Dagmar Wels1 · Roeland J. van der Schoot1 · Harry A. ten Hove1 Received: 11 January 2019 / Accepted: 26 March 2019 © The Author(s) 2019 Abstract Reef-dwelling Christmas tree worms (Spirobranchus spp.) are common coral associates. Their calcareous tubes are usually embedded in the coral skeleton and can be closed by an operculum. Tubes not overgrown by coral tissue either remain bare or become covered by algae. Despite their widespread distribution, high abundance and striking appearance, little is known about the impact of these worms on their hosts. We quantifed visible coral damage caused by Spirobranchus in Curaçao (Southern Caribbean) and found that 62.6% of worm opercula (n = 1323) caused abrasions and tissue loss in their hosts. Filamentous turf algae, known to be potentially harmful to corals, covered 76.9% of the opercula. Examination of the six most frequently inhabited host species showed a variation in the damage percentages, although this was independent of the presence of epibiotic algae on 78.4% of all opercula. Since injured corals are more susceptible to diseases, the overall nega- tive impact of Spirobranchus worms on their hosts may be more severe than previously assumed. Introduction and Nishihira 1996), even if the host becomes overgrown by sponges and octocorals, which in turn can act as replacement Coral-dwelling tubeworms of the genus Spirobranchus hosts (Hoeksema et al. 2015, 2016; García-Hernández and (Polychaeta: Serpulidae), known popularly as Christmas Hoeksema 2017). -

A Check-List of Polychaete Species from the Black
J. Black Sea/Mediterranean Environment Vol. 18, No. 1: 76-82 (2012) SHORT COMMUNICATION First sighting of the Red Sea originated stonefish (Synanceia verrucosa) from Turkey Murat Bilecenoğlu* Department of Biology, Faculty of Arts and Sciences, Adnan Menderes University, 09010, Aydın, TURKEY *Corresponding author: [email protected] Abstract A single specimen of stonefish (Synanceia verrucosa Bloch and Schneider, 1801) was recently captured off Yumurtalık (Iskenderun Bay), representing its first occurrence on the coast of Turkey and second record in the entire Mediterranean Sea. This species is famous with its highly toxic stings and possess a potential risk for human health, if it finds the opportunity to establish a successfully breeding population in the region. Key words: Synanceia verrucosa, Synanceiidae, alien species, Mediterranean Sea, Turkey Introduction The number of alien species in the Mediterranean has currently reached to the psychological threshold of 1000 species (Zenetos et al. 2010), advancing the rank of this semi-enclosed sea as one of the most invaded ecosystems on Earth. Despite of the widespread “native good, alien bad” philosophy (Goodenough 2010), ecological and sociological impacts of alien species have proven to share both sides of this approach (see Cinar et al. 2011). This paper deals with a newly introduced venomous fish species on the northeastern Levantine coast of Turkey, which doubtless takes part in the negative wing among alien biota. Species diagnosis On 18 November 2011, a single specimen of stonefish (Synanceia verrucosa Bloch and Schneider, 1801) was captured along the Yumurtalık coast (Adana, Iskenderun Bay) by an artisanal fisherman, presumably using a bottom long- line. -

PEIXES RECIFAIS DO NORDESTE BRASILEIRO.Pdf
UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS EXATAS E DA NATUREZA CURSO DE BACHARELADO EM CIÊNCIAS BIOLÓGICAS DIVERSIDADE DOS PEIXES RECIFAIS DO NORDESTE BRASILEIRO Jessé Miranda de Figueiredo Filho Ricardo de Souza Rosa Orientador João Pessoa – 2016 UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS EXATAS E DA NATUREZA CURSO DE BACHARELADO EM CIÊNCIAS BIOLÓGICAS DIVERSIDADE DOS PEIXES RECIFAIS DO NORDESTE BRASILEIRO Jessé Miranda de Figueiredo Filho Ricardo de Souza Rosa Orientador Monografia apresentada ao Curso de Ciências Biológicas como requisito parcial à obtenção do grau de Bacharel em Ciências Biológicas. João Pessoa – 2016 Catalogação na publicação Universidade Federal da Paraíba Biblioteca Setorial do CCEN Maria Teresa Macau - CRB 15/176 F475d Figueiredo Filho, Jessé Miranda de. Diversidade dos peixes recifais do Nordeste Brasileiro / Jessé Miranda de Figueiredo Filho. – João Pessoa, 2016. 40p. : il.- Monografia ( Bacharelado em Ciências Biológicas ) – Universidade Federal da Paraíba. Orientador: Profº Drº Ricardo de Souza Rosa. 1. Peixes. 2. Recifes. 3. Lista sistemática. I. Título. CDU : 597.2/.5(043.2) “By the deep sea, and music in its roar I love not man the less, but nature more.” (George Gordon Byron) AGRADECIMENTOS À minha família – em especial aos meus pais, Jessé Figueiredo e Tânia Ribeiro, por todo o apoio, confiança, carinho e conselhos que depositaram em mim durante toda a minha vida, pela ajuda nos momentos difíceis e comemorações nas alegrias da vida, sem vocês eu não teria conseguido. À minha irmã, Tayná Ribeiro, pelo apoio, sinceridade, discussões, e por sempre acreditar em mim. Ao meu orientador, Dr. Ricardo Rosa, pela orientação, paciência e confiança; por ter me apresentado ao mundo fantástico da ictiologia, e acreditado no meu potencial. -

Marine Fishes of the Azores: an Annotated Checklist and Bibliography
MARINE FISHES OF THE AZORES: AN ANNOTATED CHECKLIST AND BIBLIOGRAPHY. RICARDO SERRÃO SANTOS, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS SANTOS, RICARDO SERRÃO, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS 1997. Marine fishes of the Azores: An annotated checklist and bibliography. Arquipélago. Life and Marine Sciences Supplement 1: xxiii + 242pp. Ponta Delgada. ISSN 0873-4704. ISBN 972-9340-92-7. A list of the marine fishes of the Azores is presented. The list is based on a review of the literature combined with an examination of selected specimens available from collections of Azorean fishes deposited in museums, including the collection of fish at the Department of Oceanography and Fisheries of the University of the Azores (Horta). Personal information collected over several years is also incorporated. The geographic area considered is the Economic Exclusive Zone of the Azores. The list is organised in Classes, Orders and Families according to Nelson (1994). The scientific names are, for the most part, those used in Fishes of the North-eastern Atlantic and the Mediterranean (FNAM) (Whitehead et al. 1989), and they are organised in alphabetical order within the families. Clofnam numbers (see Hureau & Monod 1979) are included for reference. Information is given if the species is not cited for the Azores in FNAM. Whenever available, vernacular names are presented, both in Portuguese (Azorean names) and in English. Synonyms, misspellings and misidentifications found in the literature in reference to the occurrence of species in the Azores are also quoted. The 460 species listed, belong to 142 families; 12 species are cited for the first time for the Azores. -

Long-Term Effects of Snake Envenoming
toxins Review Long-Term Effects of Snake Envenoming Subodha Waiddyanatha 1,2, Anjana Silva 1,2 , Sisira Siribaddana 1 and Geoffrey K. Isbister 2,3,* 1 Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura 50008, Sri Lanka; [email protected] (S.W.); [email protected] (A.S.); [email protected] (S.S.) 2 South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, University of Peradeniya, Peradeniya 20400, Sri Lanka 3 Clinical Toxicology Research Group, University of Newcastle, Callaghan, NSW 2308, Australia * Correspondence: [email protected] or [email protected]; Tel.: +612-4921-1211 Received: 14 March 2019; Accepted: 29 March 2019; Published: 31 March 2019 Abstract: Long-term effects of envenoming compromise the quality of life of the survivors of snakebite. We searched MEDLINE (from 1946) and EMBASE (from 1947) until October 2018 for clinical literature on the long-term effects of snake envenoming using different combinations of search terms. We classified conditions that last or appear more than six weeks following envenoming as long term or delayed effects of envenoming. Of 257 records identified, 51 articles describe the long-term effects of snake envenoming and were reviewed. Disability due to amputations, deformities, contracture formation, and chronic ulceration, rarely with malignant change, have resulted from local necrosis due to bites mainly from African and Asian cobras, and Central and South American Pit-vipers. Progression of acute kidney injury into chronic renal failure in Russell’s viper bites has been reported in several studies from India and Sri Lanka. Neuromuscular toxicity does not appear to result in long-term effects.