Completion Report (MEEF 2019-002, Phase I) Value of Peri-Urban And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Recognized Villages Under the New Territories Small House Policy
LIST OF RECOGNIZED VILLAGES UNDER THE NEW TERRITORIES SMALL HOUSE POLICY Islands North Sai Kung Sha Tin Tuen Mun Tai Po Tsuen Wan Kwai Tsing Yuen Long Village Improvement Section Lands Department September 2009 Edition 1 RECOGNIZED VILLAGES IN ISLANDS DISTRICT Village Name District 1 KO LONG LAMMA NORTH 2 LO TIK WAN LAMMA NORTH 3 PAK KOK KAU TSUEN LAMMA NORTH 4 PAK KOK SAN TSUEN LAMMA NORTH 5 SHA PO LAMMA NORTH 6 TAI PENG LAMMA NORTH 7 TAI WAN KAU TSUEN LAMMA NORTH 8 TAI WAN SAN TSUEN LAMMA NORTH 9 TAI YUEN LAMMA NORTH 10 WANG LONG LAMMA NORTH 11 YUNG SHUE LONG LAMMA NORTH 12 YUNG SHUE WAN LAMMA NORTH 13 LO SO SHING LAMMA SOUTH 14 LUK CHAU LAMMA SOUTH 15 MO TAT LAMMA SOUTH 16 MO TAT WAN LAMMA SOUTH 17 PO TOI LAMMA SOUTH 18 SOK KWU WAN LAMMA SOUTH 19 TUNG O LAMMA SOUTH 20 YUNG SHUE HA LAMMA SOUTH 21 CHUNG HAU MUI WO 2 22 LUK TEI TONG MUI WO 23 MAN KOK TSUI MUI WO 24 MANG TONG MUI WO 25 MUI WO KAU TSUEN MUI WO 26 NGAU KWU LONG MUI WO 27 PAK MONG MUI WO 28 PAK NGAN HEUNG MUI WO 29 TAI HO MUI WO 30 TAI TEI TONG MUI WO 31 TUNG WAN TAU MUI WO 32 WONG FUNG TIN MUI WO 33 CHEUNG SHA LOWER VILLAGE SOUTH LANTAU 34 CHEUNG SHA UPPER VILLAGE SOUTH LANTAU 35 HAM TIN SOUTH LANTAU 36 LO UK SOUTH LANTAU 37 MONG TUNG WAN SOUTH LANTAU 38 PUI O KAU TSUEN (LO WAI) SOUTH LANTAU 39 PUI O SAN TSUEN (SAN WAI) SOUTH LANTAU 40 SHAN SHEK WAN SOUTH LANTAU 41 SHAP LONG SOUTH LANTAU 42 SHUI HAU SOUTH LANTAU 43 SIU A CHAU SOUTH LANTAU 44 TAI A CHAU SOUTH LANTAU 3 45 TAI LONG SOUTH LANTAU 46 TONG FUK SOUTH LANTAU 47 FAN LAU TAI O 48 KEUNG SHAN, LOWER TAI O 49 KEUNG SHAN, -

GEO REPORT No. 282
EXPERT REPORT ON THE GEOLOGY OF THE PROPOSED GEOPARK IN HONG KONG GEO REPORT No. 282 R.J. Sewell & D.L.K. Tang GEOTECHNICAL ENGINEERING OFFICE CIVIL ENGINEERING AND DEVELOPMENT DEPARTMENT THE GOVERNMENT OF THE HONG KONG SPECIAL ADMINISTRATIVE REGION EXPERT REPORT ON THE GEOLOGY OF THE PROPOSED GEOPARK IN HONG KONG GEO REPORT No. 282 R.J. Sewell & D.L.K. Tang This report was originally produced in June 2009 as GEO Geological Report No. GR 2/2009 2 © The Government of the Hong Kong Special Administrative Region First published, July 2013 Prepared by: Geotechnical Engineering Office, Civil Engineering and Development Department, Civil Engineering and Development Building, 101 Princess Margaret Road, Homantin, Kowloon, Hong Kong. - 3 - PREFACE In keeping with our policy of releasing information which may be of general interest to the geotechnical profession and the public, we make available selected internal reports in a series of publications termed the GEO Report series. The GEO Reports can be downloaded from the website of the Civil Engineering and Development Department (http://www.cedd.gov.hk) on the Internet. Printed copies are also available for some GEO Reports. For printed copies, a charge is made to cover the cost of printing. The Geotechnical Engineering Office also produces documents specifically for publication in print. These include guidance documents and results of comprehensive reviews. They can also be downloaded from the above website. The publications and the printed GEO Reports may be obtained from the Government’s Information Services Department. Information on how to purchase these documents is given on the second last page of this report. -

Development of a Bathing Beach at Lung Mei, Tai Po”
40/F, Revenue Tower, 5 Gloucester Road, Wan Chai, Hong Kong 香港灣仔告士打道 5 號稅務大樓 40 樓 ACE Paper 27/2008 For advice Environmental Impact Assessment Report on “Development of a Bathing Beach at Lung Mei, Tai Po” Additional Information on Ecological Surveys PURPOSE On 14 January 2008, the Advisory Council on the Environment (ACE) endorsed with conditions the Environmental Impact Assessment (EIA) report on “Development of a Bathing Beach at Lung Mei, Tai Po”. This paper provides additional information to confirm the ecological status of the habitat of Lung Mei Beach as concluded in the EIA report, addresses the conditions in the endorsement and other concerns of the ACE. ADVICE SOUGHT 2. Members are invited to note and comment on the information provided in this paper. BACKGROUND 3. The views and recommendations of the ACE on the EIA report discussed at the meeting on 14 January 2008 were set out at Annex A. METHODOLOGY OF ECOLOGICAL SURVEYS AND IMPACT ASSESSMENT UNDER THE EIA REPORT ENDORSED BY ACE WITH CONDITIONS ON 14 JANUARY 2008 4. The purpose of the ecological survey focused on collecting representative ecological data to fill information gaps concerning the following: (i) to identify the dominant and typical flora and fauna species present in the Study Area (included 500 m from the Project Site Boundary); (ii) to establish the general ecological profile, physical and ecological characteristics of the site; and (iii) to determine the presence of key factors described in Notes 1 to 3 attached to Appendix A of Annex 16 of Technical Memorandum on Environmental Impact Assessment Process (TM), including recognized sites of conservation importance, important habitats where an ecological assessment will be necessary and species of conservation importance. -
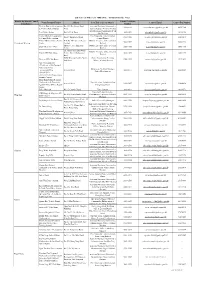
List of Access Officer (For Publication)
List of Access Officer (for Publication) - (Hong Kong Police Force) District (by District Council Contact Telephone Venue/Premise/FacilityAddress Post Title of Access Officer Contact Email Conact Fax Number Boundaries) Number Western District Headquarters No.280, Des Voeux Road Assistant Divisional Commander, 3660 6616 [email protected] 2858 9102 & Western Police Station West Administration, Western Division Sub-Divisional Commander, Peak Peak Police Station No.92, Peak Road 3660 9501 [email protected] 2849 4156 Sub-Division Central District Headquarters Chief Inspector, Administration, No.2, Chung Kong Road 3660 1106 [email protected] 2200 4511 & Central Police Station Central District Central District Police Service G/F, No.149, Queen's Road District Executive Officer, Central 3660 1105 [email protected] 3660 1298 Central and Western Centre Central District Shop 347, 3/F, Shun Tak District Executive Officer, Central Shun Tak Centre NPO 3660 1105 [email protected] 3660 1298 Centre District 2/F, Chinachem Hollywood District Executive Officer, Central Central JPC Club House Centre, No.13, Hollywood 3660 1105 [email protected] 3660 1298 District Road POD, Western Garden, No.83, Police Community Relations Western JPC Club House 2546 9192 [email protected] 2915 2493 2nd Street Officer, Western District Police Headquarters - Certificate of No Criminal Conviction Office Building & Facilities Manager, - Licensing office Arsenal Street 2860 2171 [email protected] 2200 4329 Police Headquarters - Shroff Office - Central Traffic Prosecutions Enquiry Counter Hong Kong Island Regional Headquarters & Complaint Superintendent, Administration, Arsenal Street 2860 1007 [email protected] 2200 4430 Against Police Office (Report Hong Kong Island Room) Police Museum No.27, Coombe Road Force Curator 2849 8012 [email protected] 2849 4573 Inspector/Senior Inspector, EOD Range & Magazine MT. -

GEO REPORT No. 146
FACTUAL REPORT ON HONG KONG RAINFALL AND LANDSLIDES IN 2001 GEO REPORT No. 146 T.T.M. Lam GEOTECHNICAL ENGINEERING OFFICE CIVIL ENGINEERING AND DEVELOPMENT DEPARTMENT THE GOVERNMENT OF THE HONG KONG SPECIAL ADMINISTRATIVE REGION FACTUAL REPORT ON HONG KONG RAINFALL AND LANDSLIDES IN 2001 GEO REPORT No. 146 T.T.M. Lam This report was originally produced in May 2002 as GEO Special Project Report No. SPR 2/2002 - 2 - © The Government of the Hong Kong Special Administrative Region First published, July 2004 Prepared by: Geotechnical Engineering Office, Civil Engineering and Development Department, Civil Engineering and Development Building, 101 Princess Margaret Road, Homantin, Kowloon, Hong Kong. - 3 - PREFACE In keeping with our policy of releasing information which may be of general interest to the geotechnical profession and the public, we make available selected internal reports in a series of publications termed the GEO Report series. The GEO Reports can be downloaded from the website of the Civil Engineering and Development Department (http://www.cedd.gov.hk) on the Internet. Printed copies are also available for some GEO Reports. For printed copies, a charge is made to cover the cost of printing. The Geotechnical Engineering Office also produces documents specifically for publication. These include guidance documents and results of comprehensive reviews. These publications and the printed GEO Reports may be obtained from the Government’s Information Services Department. Information on how to purchase these documents is given on the last page of this report. R.K.S. Chan Head, Geotechnical Engineering Office July 2004 - 4 - FOREWORD This report presents the factual information on rainfall and landslides in Hong Kong in 2001. -

Three Cases in China on Hakka Identity and Self-Perception
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by NORA - Norwegian Open Research Archives Three cases in China on Hakka identity and self-perception Ricky Heggheim Master’s Thesis in Chinese Studie KIN 4592, 30 Sp Departement of Culture Studies and Oriental Languages University of Oslo 1 Summary Study of Hakka culture has been an academic field for only a century. Compare with many other studies on ethnic groups in China, Hakka study and research is still in her early childhood. This despite Hakka is one of the longest existing groups of people in China. Uncertainty within the ethnicity and origin of Hakka people are among the topics that will be discussed in the following chapters. This thesis intends to give an introduction in the nature and origin of Hakka identity and to figure out whether it can be concluded that Hakka identity is fluid and depending on situations and surroundings. In that case, when do the Hakka people consider themselves as Han Chinese and when do they consider themselves as Hakka? And what are the reasons for this fluidness? Three cases in China serve as the foundation for this text. By exploring three different areas where Hakka people are settled, I hope this text can shed a light on the reasons and nature of changes in identity for Hakka people and their ethnic consciousness as well as the diversities and sameness within Hakka people in various settings and environments Conclusions that are given here indicate that Hakka people in different regions do varies in large degree when it comes to consciousness of their ethnicity and background. -

Printmgr File
IMPORTANT IMPORTANT: If you are in any doubt about this prospectus, you should obtain independent professional advice. Hongguo International Holdings Limited * (incorporated in Bermuda with limited liability) GLOBAL OFFERING Number of Offer Shares : 500,000,000 Shares (subject to the Over-allotment Option) Number of Hong Kong Offer Shares : 50,000,000 New Shares (subject to adjustment) Number of International Offer Shares : 450,000,000 Shares comprising 250,000,000 New Shares and 200,000,000 Sale Shares (subject to adjustment and the Over-allotment Option) Maximum Offer Price : HK$3.24 per Offer Share (plus brokerage of 1%, SFC transaction levy of 0.003% and Stock Exchange trading fee of 0.005%, payable in full on application subject to refund on final pricing) Nominal Value : US$0.015 per Share Stock Code : 1028 Joint Sponsors, Joint Global Coordinators and Joint Bookrunners (in alphabetical order) Joint Lead Managers Hong Kong Exchanges and Clearing Limited, The Stock Exchange of Hong Kong Limited and Hong Kong Securities Clearing Company Limited take no responsibility for the contents of this prospectus, make no representation as to its accuracy or completeness, and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this prospectus. A copy of this prospectus, having attached thereto the documents specified in Appendix VIII “Documents Delivered to the Registrar of Companies and Available for Inspection” to this prospectus, has been registered by the Registrar of Companies in Hong Kong as required by section 342C of the Companies Ordinance of Hong Kong (Chapter 32 of the Laws of Hong Kong). -

Tai Po District Council 2Nd Meeting in 2021 of the Traffic and Transport Committee
Tai Po District Council 2nd Meeting in 2021 of the Traffic and Transport Committee The 2nd meeting in 2021 of the Traffic and Transport Committee (“TTC”) under the Tai Po District Council (“TPDC”) will be held at the Conference Room, TPDC, 4/F., Tai Po Complex, 8 Heung Sze Wui Street, Tai Po at 9:30 a.m. on Friday, 7 May 2021. The agenda of the meeting is as follows: Agenda Proposed Discussion Time I. Confirmation of the minutes of the 1st meeting in 2021 of the 2 mins TTC on 5 March 2021 (9:30 – 9:32) (TPDC Paper No. TT 19/2021) II. Transport Department – Bus Route Planning Programme 60 mins 2021-2022 of Tai Po District (9:32 – 10:32) (TPDC Paper No. TT 20/2020) III. Request for the Transport Department to provide solutions to 30 mins the problems about travelling from Nai Chung Old Village to (10:32 – 11:02) Sha Tin (TPDC Paper No. TT 21/2021) IV. Proposal to maximise the use of Chuen On Road Public 30 mins Transport Interchange in Tai Po (11:02 – 11:32) (TPDC Paper No. TT 22/2020) V. Request to expand and improve Tai Wo Plaza Bus Terminus 30 mins (TPDC Paper No. TT 23/2021) (11:32 – 12:02) TT_A2_20210507_EN p.1 VI. Matters arising from the 1st meeting in 2021 of the TTC on 5 60 mins March 2021 (12:02 – 13:02) (1) Matters relating to the construction of noise barriers between Chong San Road and Tolo Highway (2) Request to improve the traffic conditions on Ting Kok Road (3) Matters relating to the traffic and bus stop facilities in the vicinity of Pak Shek Kok VII. -

TOWN PLANNING BOARD Minutes of 363Rd Meeting of the Rural And
TOWN PLANNING BOARD Minutes of 363rd Meeting of the Rural and New Town Planning Committee held at 2:30 p.m. on 14.12.2007 Present Director of Planning Chairperson Mrs. Ava S.Y. Ng Mr. Michael K.C. Lai Vice-chairman Professor Nora F.Y. Tam Mr. David W.M. Chan Mr. Edmund K.H. Leung Mr. Alfred Donald Yap Mr. B.W. Chan Mr. Y.K. Cheng Ms. Anna S.Y. Kwong Dr. James C.W. Lau Principal Environmental Protection Officer (Strategic Assessment) Environmental Protection Department Mr. H.M. Wong Deputy Director of Planning/District Secretary Mr. Lau Sing - 2 - Absent with Apologies Ms. Carmen K.M. Chan Dr. Lily Chiang Professor David Dudgeon Professor Peter R. Hills Mr. Tony C.N. Kan Dr. C.N. Ng Chief Engineer/Traffic Engineering (New Territories West), Transport Department Mr. Y.M. Lee Assistant Director (2), Home Affairs Department Ms. Margaret Hsia Assistant Director/New Territories, Lands Department Mr. C.S. Mills In Attendance Chief Town Planner/Town Planning Board Ms. Brenda K.Y. Au Town Planner/Town Planning Board Ms. Paulina L.S. PUN - 3 - Agenda Item 1 Confirmation of the Draft Minutes of the 362nd RNTPC Meeting held on 30.11.2007 [Open Meeting] 1. The draft minutes of the 362nd RNTPC meeting held on 30.11.2007 were confirmed without amendments. Agenda Item 2 Matters Arising [Open Meeting] (i) Reference of Approved Outline Zoning Plan 2. The Secretary reported that on 4.12.2007, the Chief Executive in Council referred the approved Peng Chau Outline Zoning Plan to the Town Planning Board for amendment under section 12(1)(b)(ii) of the Town Planning Ordinance. -

New Territories Urban Route No. 74A TAI WO to KWUN TONG FERRY
L. S. NO. 2 TO GAZETTE NO. 12/2001L.N. 71 of 2001 B463 New Territories Urban Route No. 74A TAI WO to KWUN TONG FERRY: via Po Nga Road, Ting Kok Road, On Chee Road, On Cheung Road, Po Heung Bridge, Po Heung Street, Plover Cove Road, Nam Wan Road, Kwong Fuk Road, Wan Tau Street, Nam Wan Road, Tat Wan Road, Wan Tau Tong Estate Bus Terminus, Tat Wan Road, Nam Wan Road, Tai Po Road, Yuen Wo Road, Fo Tan Road, *(Tai Chung Kiu Road, Lion Rock Tunnel Road), Lion Rock Tunnel, Lung Cheung Road, Po Kong Village Road Interchange, Po Kong Village Road, Choi Hung Road, Choi Hung access road, Prince Edward Road East, Kwun Tong Road, Hoi Yuen Road and Kwun Tong Ferry Concourse access road. * Journeys may be diverted via Tai Chung Kiu Road, Che Kung Miu Road, Hung Mui Kuk Road and Lion Rock Tunnel Road non-stop during 7.30 a.m. to 9.30 a.m. and during 4.00 p.m. to 7.00 p.m. on days except Sundays and public holidays depending on traffic condition. KWUN TONG FERRY to TAI WO: via Wai Yip Street, King Yip Street, *(Cha Kwo Ling Road, Lei Yue Mun Road,) Kwun Tong Road, Lung Cheung Road, Hammer Hill Road, Choi Hung Road, Choi Hung Temporary Bus Terminus, Choi Hung Road, Hammer Hill Road, Lung Cheung Road, Lion Rock Tunnel, Lion Rock Tunnel Road, Tai Chung Kiu Road, Fo Tan Road, Yuen Wo Road, Tai Po Road, Nam Wan Road, Tat Wan Road, Wan Tau Tong Estate Bus Terminus, Tat Wan Road, Nam Wan Road, Wan Tau Street, Heung Sze Wui Street, Po Heung Street, Po Heung Bridge, On Cheung Road, On Chee Road, Ting Kok Road, Tai Po Tai Wo Road and Po Nga Road. -

Introduction
INTRODUCTION 1) Background In many people’s minds, Hong Kong is the “Pearl of the Orient” and a compact cosmopolitan city of skyscrapers and shopping arcades. Whether it is the foreign tourists or the locals, they always envision activities like shopping and sightseeing as the tourists’ ultimate activities. Renowned landmarks like the Peak and Tsing Ma Bridge are seen as the must-goes and final stops on their itineraries. However, few seem to realize the city has its peculiar and exotic side. With its rich cultural diversity, more than 260 outlying islands, 100 walking trails, 4 marine parks, Hong Kong promises wonderful landscapes, dramatic and stunning rock formations, craggy mountains, quaint villages, inviting flora and fauna, abundant cultural heritage which cater for not only the interests of the shoppers but also the most adventurous explorers. It is well worth the efforts to explore these charming rural retreats in Hong Kong. The aim of this project is to examine these adventurous places in Hong Kong. As there are many hotels tourists can choose from in Tsim Sha Tsui, we will start our exploration of these adventurous places from there. 1 B) Definitions and our understanding of Adventure Tourism Definitions According to the information given by Queensland Tourism Board, adventure tourism can be classified into two main categories, namely i) Hard adventure tourism ii) Soft adventure tourism There is a distinction between the two kinds of adventure tourism. For hard adventure tourism, it combines a unique experience in an outdoor setting with excitement and a degree of risk. It frequently demands physical exertion as well as a level of skill. -

District : Tai Po
District : Tai Po Proposed District Council Constituency Areas +/- % of Population Projected Quota Code Proposed Name Boundary Description Major Estates/Areas Population (16 599) P01 Tai Po Hui 19 451 +17.18 N Plover Cove Road, Po Heung Bridge 1. JADE GARDEN 2. MOUNTAIN VIEW COURT Po Heung Street 3. PO HEUNG ESTATE NE Plover Cove Road E Nam Wan Road, Tai Po River Tai Po Road - Yuen Chau Tsai SE MTR(East Rail Line), Nam Wan Road S MTR(East Rail Line) SW MTR(East Rail Line) W MTR(East Rail Line) NW Lam Tsuen River, MTR(East Rail Line) Pak Shing Street P02 Chung Ting 14 303 -13.83 N Ting Lai Road 1. CHUNG NGA COURT 2. EIGHTLAND GARDENS NE Chung Nga Road 3. FORTUNE PLAZA E On Cheung Road, Ting Kok Road 4. FU HENG ESTATE (PART) : Heng Tai House SE Lam Tsuen River, On Chee Road, On Po Road 5. JADE PLAZA S Lam Tsuen River 6. TING NGA COURT 7. TREASURE GARDEN SW Lam Tsuen River, Ting Kok Road W Ting Kok Road NW Ting Lai Road P 1 District : Tai Po Proposed District Council Constituency Areas +/- % of Population Projected Quota Code Proposed Name Boundary Description Major Estates/Areas Population (16 599) P03 Tai Po Central 13 645 -17.80 N On Po Road, On Tai Road 1. TAI PO CENTRE 2. TAI PO PLAZA NE Nam Wan Road, On Po Road E Nam Wan Road SE Lam Tsuen River, Nam Wan Road S Lam Tsuen River SW Lam Tsuen River, Po Wu Lane Tai Po Tai Wo Road W On Chee Road, On Po Road, On Pong Road NW P04 Tai Yuen 13 863 -16.48 N Ting Kok Road 1.