Do Natural Differences in Acoustic Signals Really Interfere in Conspecific Recognition in the Pan-Amazonian Frog Allobates Femoralis?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
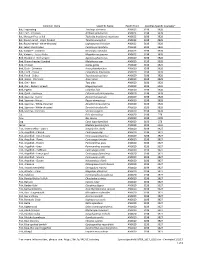
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

Amphibia, Anura, Dendrobatidae, Allobates Femoralis (Boulenger, 1884): First Confirmed Country Records, Venezuela
ISSN 1809-127X (online edition) © 2010 Check List and Authors Chec List Open Access | Freely available at www.checklist.org.br Journal of species lists and distribution N Allobates femoralis ISTRIBUTIO Amphibia, Anura, Dendrobatidae, D Venezuela (Boulenger, 1884): First confirmed country records, RAPHIC G César L. Barrio-Amorós and Juan Carlos Santos 2 EO 1* G N O 1 Fundación Andígena, [email protected] postal 210, 5101-A. Mérida, Venezuela. OTES 2 University of Texas at Austin, Integrative Biology. 1 University Station C0930. Austin, TX 78705, USA. N * Corresponding author E-mail: Abstract: Allobates femoralis analized advertisement calls taken at two Venezuelan localities. The presence of the dendrobatid frog in Venezuela is herein confirmed though recorded and Allobates femoralis is a common dendrobatid frog widely distributed throughout the Amazon and Guianas in Colombia, Ecuador, Peru, Bolivia, Brazil, French Guiana, series of four notes at Las Claritas is 0.52 sec; the dominant Suriname and Guyana (Lötters et al. frequency is at 2954 Hz and the fundamental frequency is error, based on KU (Natural History 2007). Museum, The firstUniversity report of this species from Venezuela (Duellman 1997) was in was later recognized as Ameerega guayanensis (Barrio- Amorósof Kansas, 2004). Lawrence, Ameerega Kansas, guayanensisUSA) 167335. was This describedspecimen 2004).for populations Today however, of eastern this Venezuelataxon validity and is some questionable authors considered it as valid (Schulte 1999; Barrio-Amorós A.(J.C. (picta) Santos guayanensis unpublished because data). itSince is theno validnomenclatural available act was yet performed, we herein use the combinationAllobates femoralis was removed from the Venezuelan checklist name for those populations. -

Acoustic Interference and Recognition Space Within a Complex Assemblage of Dendrobatid Frogs
Acoustic interference and recognition space within a complex assemblage of dendrobatid frogs Adolfo Amézquitaa,1, Sandra Victoria Flechasa, Albertina Pimentel Limab, Herbert Gasserc, and Walter Hödlc aDepartment of Biological Sciences, Universidad de los Andes, Bogotá, AA 4976, Colombia; bInstituto Nacional de Pesquisas da Amazônia, CEP 69060-001, Manaus, Brazil; and cDepartment of Evolutionary Biology, Institute of Zoology, University of Vienna, A-1090 Vienna, Austria Edited by Michael J. Ryan, University of Texas at Austin, Austin, TX, and accepted by the Editorial Board September 1, 2011 (received for review March 24, 2011) In species-rich assemblages of acoustically communicating animals, insects (22), and frogs (23, 24). Because heterospecific sounds may heterospecific sounds may constrain not only the evolution of sig- represent noise that interferes with signal detection (25, 26), the nal traits but also the much less-studied signal-processing mecha- communication systems should reveal adaptations that reduce its nisms that define the recognition space of a signal. To test the effects. To date, most research to validate this idea has been hypothesis that the recognition space is optimally designed, i.e., conducted on the characteristics of the signals or on the pattern of that it is narrower toward the species that represent the higher signaling activity (27, 28), but compelling evidence suggests that potential for acoustic interference, we studied an acoustic assem- recognition systems can evolve without concomitant variation in blage of 10 diurnally active frog species. We characterized their the signals (20, 29). We present here a manipulative study on a calls, estimated pairwise correlations in calling activity, and, to species-rich assemblage of diurnal frogs to test the general model the recognition spaces of five species, conducted playback hypothesis that the recognition space is shaped in a way that experiments with 577 synthetic signals on 531 males. -

Decoupled Evolution Between Senders and Receivers in the Neotropical Allobates Femoralis Frog Complex
RESEARCH ARTICLE Decoupled Evolution between Senders and Receivers in the Neotropical Allobates femoralis Frog Complex Mileidy Betancourth-Cundar1*, Albertina P. Lima2, Walter Hödl3, Adolfo Amézquita1 1 Department of Biological Sciences, Universidad de Los Andes, Bogotá, Colombia, 2 Coordenaçãode Pesquisas em Biodiversidade, Instituto Nacional de Pesquisas da Amazônia, Manaus, Amazonas, Brazil, 3 Department of Integrative Zoology, University of Vienna, Vienna, Austria a11111 * [email protected] Abstract During acoustic communication, an audible message is transmitted from a sender to a OPEN ACCESS receiver, often producing changes in behavior. In a system where evolutionary changes of the sender do not result in a concomitant adjustment in the receiver, communication and Citation: Betancourth-Cundar M, Lima AP, Hödl W, species recognition could fail. However, the possibility of an evolutionary decoupling Amézquita A (2016) Decoupled Evolution between Senders and Receivers in the Neotropical Allobates between sender and receiver has rarely been studied. Frog populations in the Allobates femoralis Frog Complex. PLoS ONE 11(6): femoralis cryptic species complex are known for their extensive morphological, genetic and e0155929. doi:10.1371/journal.pone.0155929 acoustic variation. We hypothesized that geographic variation in acoustic signals of A. Editor: William J. Etges, University of Arkansas, femoralis was correlated with geographic changes in communication through changes in UNITED STATES male-male recognition. To test -

1704632114.Full.Pdf
Phylogenomics reveals rapid, simultaneous PNAS PLUS diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary Yan-Jie Fenga, David C. Blackburnb, Dan Lianga, David M. Hillisc, David B. Waked,1, David C. Cannatellac,1, and Peng Zhanga,1 aState Key Laboratory of Biocontrol, College of Ecology and Evolution, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510006, China; bDepartment of Natural History, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611; cDepartment of Integrative Biology and Biodiversity Collections, University of Texas, Austin, TX 78712; and dMuseum of Vertebrate Zoology and Department of Integrative Biology, University of California, Berkeley, CA 94720 Contributed by David B. Wake, June 2, 2017 (sent for review March 22, 2017; reviewed by S. Blair Hedges and Jonathan B. Losos) Frogs (Anura) are one of the most diverse groups of vertebrates The poor resolution for many nodes in anuran phylogeny is and comprise nearly 90% of living amphibian species. Their world- likely a result of the small number of molecular markers tra- wide distribution and diverse biology make them well-suited for ditionally used for these analyses. Previous large-scale studies assessing fundamental questions in evolution, ecology, and conser- used 6 genes (∼4,700 nt) (4), 5 genes (∼3,800 nt) (5), 12 genes vation. However, despite their scientific importance, the evolutionary (6) with ∼12,000 nt of GenBank data (but with ∼80% missing history and tempo of frog diversification remain poorly understood. data), and whole mitochondrial genomes (∼11,000 nt) (7). In By using a molecular dataset of unprecedented size, including 88-kb the larger datasets (e.g., ref. -

Methodological Development for Harmonic Direction Finder Tracking in Salamanders
Herpetological Conservation and Biology 13(2):473–478. Submitted: 3 April 2017; Accepted: 6 June 2018; Published: 31 August 2018. METHODOLOGICAL DEVELOPMENT FOR HARMONIC DIRECTION FINDER TRACKING IN SALAMANDERS AMAËL BORZÉE1,2, YE INN KIM2, KYUNGMIN KIM3, AND YIKWEON JANG2,3,4 1Laboratory of Behavioral Ecology and Evolution, School of Biological Sciences, Seoul National University, 08826, Republic of Korea 2Division of EcoScience, Ewha Womans University, Seoul 03760, Republic of Korea 3Interdisciplinary Program of EcoCreative, Ewha Womans University, Seoul 03760, Republic of Korea 4Corresponding author, e-mail: [email protected] Abstract.—The ability to track the movements of an animal enables a better understanding of its behavior and ecological preferences. However, animals that are small, have smooth sensitive skin, and live in variable environments can be difficult to track. We tested several types of Harmonic Detection Finder (HDF) tags and harnesses in salamanders. We designed a harness that does not hinder the normal movements of a salamander and is difficult to remove for the bearer. We also designed a dipole that could be used both on the ground and underwater without a great decrease in detection range. These techniques are important for non-invasive behavioral studies, although the harness developed here could easily get entangled in narrow or heavily vegetated habitats. Key Words.—caudata; Harmonic Direction Finder; harness; tracking INTRODUCTION faber; Oliveira et al. 2016), Suweon Treefrogs (Dryophytes suweonensis), and Japanese Treefrogs (D. Tracking animals to understand their behavior is as japonicus; Borzée et al. 2016). old as primitive hunting practices, but the first report of Tracking salamanders has relied primarily on tracking for scientific information dates back to 1803 subcutaneous implants, mostly because of the preference when banding was used to demonstrate philopatry in of the species for narrow hiding refuges. -

Widespread Occurrence of Bd in French Guiana, South America
RESEARCH ARTICLE Widespread Occurrence of Bd in French Guiana, South America Elodie A. Courtois1,2*, Philippe Gaucher2, Jérôme Chave3, Dirk S. Schmeller4,5,6 1 Station d'Ecologie Expérimentale du Centre National de la Recherche Scientifique (CNRS) à Moulis, Unité de Service et de Recherche (USR) 2936, Saint Girons, France, 2 Centre National de la Recherche Scientifique Guyane, Unité de Service et de Recherche (USR) 3456, Cayenne, Guyane Française, 3 Laboratoire Ecologie et Diversité Biologique, Unité Mixte de Recherche (UMR) 5174, Toulouse, France, 4 Helmholtz-Centre for Environmental Research, Department of Conservation Biology, Leipzig, Germany, 5 Université de Toulouse, Laboratoire Ecologie Fonctionnelle et Environnement (Ecolab), Toulouse, France, a11111 6 Centre National de la Recherche Scientifique, EcoLab, Toulouse, France * [email protected] Abstract OPEN ACCESS The amphibian chytrid fungus Batrachochytrium dendrobatidis (Bd) is a purported agent of decline and extinction of many amphibian populations worldwide. Its occurrence remains Citation: Courtois EA, Gaucher P, Chave J, ’ Schmeller DS (2015) Widespread Occurrence of Bd poorly documented in many tropical regions, including the Guiana Shield, despite the area s in French Guiana, South America. PLoS ONE 10(4): high amphibian diversity. We conducted a comprehensive assessment of Bd in French Gui- e0125128. doi:10.1371/journal.pone.0125128 ana in order to (1) determine its geographical distribution, (2) test variation of Bd prevalence Academic Editor: Matthew C Fisher, Imperial among species in French Guiana and compare it to earlier reported values in other South College Faculty of Medicine, UNITED KINGDOM American anuran species (http://www.bd-maps.net; 123 species from 15 genera) to define Received: September 10, 2014 sentinel species for future work, (3) track changes in prevalence through time and (4) deter- Accepted: March 11, 2015 mine if Bd presence had a negative effect on one selected species. -

The Amphibians of the Mitaraka Massif, French Guiana
DIRECTEUR DE LA PUBLICATION : Bruno David Président du Muséum national d’Histoire naturelle RÉDACTRICE EN CHEF / EDITOR-IN-CHIEF : Laure Desutter-Grandcolas ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Anne Mabille ([email protected]), Emmanuel Côtez MISE EN PAGE / PAGE LAYOUT : Anne Mabille COMITÉ SCIENTIFIQUE / SCIENTIFIC BOARD : James Carpenter (AMNH, New York, États-Unis) Maria Marta Cigliano (Museo de La Plata, La Plata, Argentine) Henrik Enghoff (NHMD, Copenhague, Danemark) Rafael Marquez (CSIC, Madrid, Espagne) Peter Ng (University of Singapore) Norman I. Platnick (AMNH, New York, États-Unis) Jean-Yves Rasplus (INRA, Montferrier-sur-Lez, France) Jean-François Silvain (IRD, Gif-sur-Yvette, France) Wanda M. Weiner (Polish Academy of Sciences, Cracovie, Pologne) John Wenzel (The Ohio State University, Columbus, États-Unis) COUVERTURE / COVER : Flooded bank of Alama river in the Mitaraka Mountains (French Guiana) (photo Marc Pollet). In medaillon, Synapturanus cf. mirandaribeiroi Nelson & Lescure, 1975. Zoosystema est indexé dans / Zoosystema is indexed in: – Science Citation Index Expanded (SciSearch®) – ISI Alerting Services® – Current Contents® / Agriculture, Biology, and Environmental Sciences® – Scopus® Zoosystema est distribué en version électronique par / Zoosystema is distributed electronically by: – BioOne® (http://www.bioone.org) Les articles ainsi que les nouveautés nomenclaturales publiés dans Zoosystema sont référencés par / Articles and nomenclatural novelties published in Zoosystema are referenced by: – ZooBank® (http://zoobank.org) Zoosystema est une revue en flux continu publiée par les Publications scientifiques du Muséum, Paris / Zoosystema is a fast track journal published by the Museum Science Press, Paris Les Publications scientifiques du Muséum publient aussi / The Museum Science Press also publish: Adansonia, Geodiversitas, Anthropozoologica, European Journal of Taxonomy, Naturae, Cryptogamie sous-sections Algologie, Bryologie, Mycologie. -

The Influence of Internote-Interval Variation of the Advertisement Call
Amphibia-Reptilia 28 (2007): 227-234 The influence of internote-interval variation of the advertisement call on the phonotactic behaviour in male Allobates femoralis (Dendrobatidae) Mario Göd, Alexander Franz, Walter Hödl* Abstract. Acoustic conspecific recognition depends on spectral and temporal call properties, but not all of these are necessary for species identification. To test the importance of the advertisement call’s internote interval of the dendrobatid frog Allobates (=Epipedobates) femoralis, we conducted field playback experiments with modified conspecific calls and investigated phonotactic responses. Allobates femoralis males showed positive phonotaxis when calls with internote intervals varying up to ±60% of the population’s mean were presented. No phonotactic approach was observed in response to modified advertisement calls containing internote intervals of the syntopic heterospecific leptodactylid frog Adenomera hylaedactyla. However, 26.7% of the A. femoralis males tested showed positive phototoxic when altered advertisement calls with internote intervals of the syntopic heterospecific dendrobatid frog Epipedobates cf. hahneli were presented. Our results show that only a combination of different acoustic call parameters provides correct species-specific recognition. Introduction Pyles, 1983; Schwartz and Wells, 1984; Garcia- Rutledge and Narins, 2001). Nevertheless, even Beside the attraction of conspecific females as when the means differ, the distributions of mating partners, the function of male anuran the properties of different -

Review of Non-Cites Amphibia Species That Are Known Or Likely to Be in International Trade
REVIEW OF NON-CITES AMPHIBIA SPECIES THAT ARE KNOWN OR LIKELY TO BE IN INTERNATIONAL TRADE (Version edited for public release) Prepared for the European Commission Directorate General E - Environment ENV.E.2. – Development and Environment by the United Nations Environment Programme World Conservation Monitoring Centre November, 2007 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK ABOUT UNEP WORLD CONSERVATION MONITORING CENTRE www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme (UNEP), the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognize the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The European Commission, Brussels, Belgium Prepared by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. The designations employed and the presentations do not imply the expressions of any opinion whatsoever on the part of UNEP, the European Commission or contributory organisations concerning the legal status of any country, territory, city or area or its authority, or concerning the delimitation of its frontiers or boundaries.