Population Regulation of Frogs and Lizards in a Lowland Wet Neotropical Forest: Integrating Alternative Hypotheses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Self-Repair and Self-Cleaning of the Lepidopteran Proboscis
Clemson University TigerPrints All Dissertations Dissertations 8-2019 Self-Repair and Self-Cleaning of the Lepidopteran Proboscis Suellen Floyd Pometto Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Pometto, Suellen Floyd, "Self-Repair and Self-Cleaning of the Lepidopteran Proboscis" (2019). All Dissertations. 2452. https://tigerprints.clemson.edu/all_dissertations/2452 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. SELF-REPAIR AND SELF-CLEANING OF THE LEPIDOPTERAN PROBOSCIS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy ENTOMOLOGY by Suellen Floyd Pometto August 2019 Accepted by: Dr. Peter H. Adler, Major Advisor and Committee Co-Chair Dr. Eric Benson, Committee Co-Chair Dr. Richard Blob Dr. Patrick Gerard i ABSTRACT The proboscis of butterflies and moths is a key innovation contributing to the high diversity of the order Lepidoptera. In addition to taking nectar from angiosperm sources, many species take up fluids from overripe or sound fruit, plant sap, animal dung, and moist soil. The proboscis is assembled after eclosion of the adult from the pupa by linking together two elongate galeae to form one tube with a single food canal. How do lepidopterans maintain the integrity and function of the proboscis while foraging from various substrates? The research questions included whether lepidopteran species are capable of total self- repair, how widespread the capability of self-repair is within the order, and whether the repaired proboscis is functional. -

The Predatory Mite (Acari, Parasitiformes: Mesostigmata (Gamasina); Acariformes: Prostigmata) Community in Strawberry Agrocenosis
Acta Universitatis Latviensis, Biology, 2004, Vol. 676, pp. 87–95 The predatory mite (Acari, Parasitiformes: Mesostigmata (Gamasina); Acariformes: Prostigmata) community in strawberry agrocenosis Valentîna Petrova*, Ineta Salmane, Zigrîda Çudare Institute of Biology, University of Latvia, Miera 3, Salaspils LV-2169, Latvia *Corresponding author, E-mail: [email protected]. Abstract Altogether 37 predatory mite species from 14 families (Parasitiformes and Acariformes) were collected using leaf sampling and pit-fall trapping in strawberry fi elds (1997 - 2001). Thirty- six were recorded on strawberries for the fi rst time in Latvia. Two species, Paragarmania mali (Oud.) (Aceosejidae) and Eugamasus crassitarsis (Hal.) (Parasitidae) were new for the fauna of Latvia. The most abundant predatory mite families (species) collected from strawberry leaves were Phytoseiidae (Amblyseius cucumeris Oud., A. aurescens A.-H., A. bicaudus Wainst., A. herbarius Wainst.) and Anystidae (Anystis baccarum L.); from pit-fall traps – Parasitidae (Poecilochirus necrophori Vitz. and Parasitus lunaris Berl.), Aceosejidae (Leioseius semiscissus Berl.) and Macrochelidae (Macrocheles glaber Müll). Key words: agrocenosis, diversity, predatory mites, strawberry. Introduction Predatory mites play an important ecological role in terrestrial ecosystems and they are increasingly being used in management for biocontrol of pest mites, thrips and nematodes (Easterbrook 1992; Wright, Chambers 1994; Croft et al. 1998; Cuthbertson et al. 2003). Many of these mites have a major infl uence on nutrient cycling, as they are predators on other arthropods (Santos 1985; Karg 1993; Koehler 1999). In total, investigations of mite fauna in Latvia were made by Grube (1859), who found 28 species, Eglītis (1954) – 50 species, Kuznetsov and Petrov (1984) – 85 species, Lapiņa (1988) – 207 species, and Salmane (2001) – 247 species. -

A Collection of Amphibians from Río San Juan, Southeastern Nicaragua
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/264789493 A collection of amphibians from Río San Juan, southeastern Nicaragua Article in Herpetology Notes · January 2009 CITATIONS READS 12 188 4 authors, including: Javier Sunyer Matthias Dehling University of Canterbury 89 PUBLICATIONS 209 CITATIONS 54 PUBLICATIONS 967 CITATIONS SEE PROFILE SEE PROFILE Gunther Köhler Senckenberg Research Institute 222 PUBLICATIONS 1,617 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Zoological Research in Strict Forest Reserves in Hesse, Germany View project Diploma Thesis View project All content following this page was uploaded by Javier Sunyer on 16 August 2018. The user has requested enhancement of the downloaded file. Herpetology Notes, volume 2: 189-202 (2009) (published online on 29 October 2009) A collection of amphibians from Río San Juan, southeastern Nicaragua Javier Sunyer1,2,3*, Guillermo Páiz4, David Matthias Dehling1, Gunther Köhler1 Abstract. We report upon the amphibians collected during seven expeditions carried out between the years 2000–2006 to thirteen localities in both Refugio de Vida Silvestre Río San Juan and Reserva Biológica Indio-Maíz, southeastern Nicaragua. We include morphometric data of around one-half of the adult specimens in the collection, and provide a brief general overview and discuss zoogeographic and conservation considerations of the amphibians known to occur in the Río San Juan area. Keywords. Amphibia, conservation, ecology, morphometry, zoogeography. Introduction potential of holding America’s first interoceanic channel and also because it was part of the sea route to travel The San Juan River is an approximately 200 km slow- from eastern to western United States. -

Community Structure of Mites (Acari: Acariformes and Parasitiformes) in Nests of the Semi-Collared Flycatcher (Ficedula Semitorquata) R
International Research Journal of Natural Sciences Vol.3, No.3, pp.48-53, December 2015 ___Published by European Centre for Research Training and Development UK (www.eajournals.org) COMMUNITY STRUCTURE OF MITES (ACARI: ACARIFORMES AND PARASITIFORMES) IN NESTS OF THE SEMI-COLLARED FLYCATCHER (FICEDULA SEMITORQUATA) R. Davidova, V. Vasilev, N. Ali, J. Bakalova Konstantin Preslavsky University of Shumen, 115, Universitetska Str., Shumen, 9700, Bulgaria. ABSTRACT: The aims of the present paper are to establish the specific structure of communities of prostigmatic and mesostigmatic mites in nests of the semi-collared flycatcher (Ficedula semitorquata) and to compare the fauna with the mites in nests of two other European flycatchers. For analysis of community structure of mites were used the indices: prevalence, relative density, mean intensity and dominance. Mite communities are strongly dominated by the species Dermanyssus gallinae and Ornithonyssus sylviarum, which were found with the highest frequency and dominance. The mite communities are characterized by a large number of subrecedent species. KEYWORDS: Acariformes, Parasitiformes, Nest of Bird, Community Structure INTRODUCTION The nests of different species of birds are an example of a fairly unstable and isolated habitat, with its own dependent on it specific fauna which involves different groups of invertebrate animals. One of the components of this fauna which demonstrates particular abundance is the arthropods, and more specifically, the mites. The studies of Parasitiformes show that mesostigmatic mites living in birds' nests vary both in terms of their species affiliation and the structure of their communities [4, 8]. Highly important with respect to veterinary science and medicine are a number of species, such as Ornithonyssus bursa, Ornithonyssus sylviarum, Dermanyssus gallinae harboured by birds, Ornithonyssus bacoti, harboured by rodents, etc. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Species Delimitation in Asexual Insects of Economic Importance: the Case of Black Scale (Parasaissetia Nigra), a Cosmopolitan Parthenogenetic Pest Scale Insect
RESEARCH ARTICLE Species delimitation in asexual insects of economic importance: The case of black scale (Parasaissetia nigra), a cosmopolitan parthenogenetic pest scale insect Yen-Po Lin1,2,3*, Robert D. Edwards4, Takumasa Kondo5, Thomas L. Semple3, Lyn G. Cook2 a1111111111 1 College of Life Science, Shanxi University, Taiyuan, Shanxi, China, 2 School of Biological Sciences, The University of Queensland, Brisbane, Queensland, Australia, 3 Research School of Biology, Division of a1111111111 Evolution, Ecology and Genetics, The Australian National University, Canberra, Australian Capital Territory, a1111111111 Australia, 4 Department of Botany, National Museum of Natural History, Smithsonian Institution, Washington a1111111111 DC, United States of America, 5 CorporacioÂn Colombiana de InvestigacioÂn Agropecuaria (CORPOICA), a1111111111 Centro de InvestigacioÂn Palmira, Valle del Cauca, Colombia * [email protected] OPEN ACCESS Abstract Citation: Lin Y-P, Edwards RD, Kondo T, Semple TL, Cook LG (2017) Species delimitation in asexual Asexual lineages provide a challenge to species delimitation because species concepts insects of economic importance: The case of black either have little biological meaning for them or are arbitrary, since every individual is mono- scale (Parasaissetia nigra), a cosmopolitan phyletic and reproductively isolated from all other individuals. However, recognition and parthenogenetic pest scale insect. PLoS ONE 12 naming of asexual species is important to conservation and economic applications. Some (5): e0175889. https://doi.org/10.1371/journal. pone.0175889 scale insects are widespread and polyphagous pests of plants, and several species have been found to comprise cryptic species complexes. Parasaissetia nigra (Nietner, 1861) Editor: Wolfgang Arthofer, University of Innsbruck, AUSTRIA (Hemiptera: Coccidae) is a parthenogenetic, cosmopolitan and polyphagous pest that feeds on plant species from more than 80 families. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
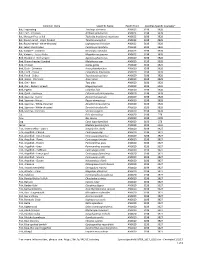
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

Amphibia, Anura, Dendrobatidae, Allobates Femoralis (Boulenger, 1884): First Confirmed Country Records, Venezuela
ISSN 1809-127X (online edition) © 2010 Check List and Authors Chec List Open Access | Freely available at www.checklist.org.br Journal of species lists and distribution N Allobates femoralis ISTRIBUTIO Amphibia, Anura, Dendrobatidae, D Venezuela (Boulenger, 1884): First confirmed country records, RAPHIC G César L. Barrio-Amorós and Juan Carlos Santos 2 EO 1* G N O 1 Fundación Andígena, [email protected] postal 210, 5101-A. Mérida, Venezuela. OTES 2 University of Texas at Austin, Integrative Biology. 1 University Station C0930. Austin, TX 78705, USA. N * Corresponding author E-mail: Abstract: Allobates femoralis analized advertisement calls taken at two Venezuelan localities. The presence of the dendrobatid frog in Venezuela is herein confirmed though recorded and Allobates femoralis is a common dendrobatid frog widely distributed throughout the Amazon and Guianas in Colombia, Ecuador, Peru, Bolivia, Brazil, French Guiana, series of four notes at Las Claritas is 0.52 sec; the dominant Suriname and Guyana (Lötters et al. frequency is at 2954 Hz and the fundamental frequency is error, based on KU (Natural History 2007). Museum, The firstUniversity report of this species from Venezuela (Duellman 1997) was in was later recognized as Ameerega guayanensis (Barrio- Amorósof Kansas, 2004). Lawrence, Ameerega Kansas, guayanensisUSA) 167335. was This describedspecimen 2004).for populations Today however, of eastern this Venezuelataxon validity and is some questionable authors considered it as valid (Schulte 1999; Barrio-Amorós A.(J.C. (picta) Santos guayanensis unpublished because data). itSince is theno validnomenclatural available act was yet performed, we herein use the combinationAllobates femoralis was removed from the Venezuelan checklist name for those populations. -

Identification of Trombiculid Chigger Mites Collected on Rodents from Southern Vietnam and Molecular Detection of Rickettsiaceae Pathogen
ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 Korean J Parasitol Vol. 58, No. 4: 445-450, August 2020 ▣ ORIGINAL ARTICLE https://doi.org/10.3347/kjp.2020.58.4.445 Identification of Trombiculid Chigger Mites Collected on Rodents from Southern Vietnam and Molecular Detection of Rickettsiaceae Pathogen 1, 2, 1 3 4,5, 4,5, Minh Doan Binh †, Sinh Cao Truong †, Dong Le Thanh , Loi Cao Ba , Nam Le Van * , Binh Do Nhu * 1Ho Chi Minh Institute of Malariology-Parasitology and Entomology, Ho Chi Minh Vietnam; 2Vinh Medical University, Nghe An, Vietnam; 3National Institute of Malariology-Parasitology and Entomology, Ha Noi, Vietnam; 4Military Hospital 103, Ha Noi, Vietnam; 5Vietnam Military Medical University, Ha Noi, Vietnam Abstract: Trombiculid “chigger” mites (Acari) are ectoparasites that feed blood on rodents and another animals. A cross- sectional survey was conducted in 7 ecosystems of southern Vietnam from 2015 to 2016. Chigger mites were identified with morphological characteristics and assayed by polymerase chain reaction for detection of rickettsiaceae. Overall chigger infestation among rodents was 23.38%. The chigger index among infested rodents was 19.37 and a mean abun- dance of 4.61. A total of 2,770 chigger mites were identified belonging to 6 species, 3 genera, and 1 family, and pooled into 141 pools (10-20 chiggers per pool). Two pools (1.4%) of the chiggers were positive for Orientia tsutsugamushi. Rick- etsia spp. was not detected in any pools of chiggers. Further studies are needed including a larger number and diverse hosts, and environmental factors to assess scrub typhus. Key words: Oriental tsutsugamushi, Rickettsia sp., chigger mite, ectoparasite INTRODUCTION Orientia tsutsugamushi is a gram-negative bacteria and caus- ative agent of scrub typhus, is a vector-borne zoonotic disease Trombiculid mites (Acari: Trombiculidae) are ectoparasites with the potential of causing life-threatening febrile infection that are found in grasses and herbaceous vegetation. -

Trophobiosis Between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): an Overview
December, 2001 Neotropical Entomology 30(4) 501 FORUM Trophobiosis Between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): an Overview JACQUES H.C. DELABIE 1Lab. Mirmecologia, UPA Convênio CEPLAC/UESC, Centro de Pesquisas do Cacau, CEPLAC, C. postal 7, 45600-000, Itabuna, BA and Depto. Ciências Agrárias e Ambientais, Univ. Estadual de Santa Cruz, 45660-000, Ilhéus, BA, [email protected] Neotropical Entomology 30(4): 501-516 (2001) Trofobiose Entre Formicidae e Hemiptera (Sternorrhyncha e Auchenorrhyncha): Uma Visão Geral RESUMO – Fêz-se uma revisão sobre a relação conhecida como trofobiose e que ocorre de forma convergente entre formigas e diferentes grupos de Hemiptera Sternorrhyncha e Auchenorrhyncha (até então conhecidos como ‘Homoptera’). As principais características dos ‘Homoptera’ e dos Formicidae que favorecem as interações trofobióticas, tais como a excreção de honeydew por insetos sugadores, atendimento por formigas e necessidades fisiológicas dos dois grupos de insetos, são discutidas. Aspectos da sua evolução convergente são apresenta- dos. O sistema mais arcaico não é exatamente trofobiótico, as forrageadoras coletam o honeydew despejado ao acaso na folhagem por indivíduos ou grupos de ‘Homoptera’ não associados. As relações trofobióticas mais comuns são facultativas, no entanto, esta forma de mutualismo é extremamente diversificada e é responsável por numerosas adaptações fisiológicas, morfológicas ou comportamentais entre os ‘Homoptera’, em particular Sternorrhyncha. As trofobioses mais diferenciadas são verdadeiras simbioses onde as adaptações mais extremas são observadas do lado dos ‘Homoptera’. Ao mesmo tempo, as formigas mostram adaptações comportamentais que resultam de um longo período de coevolução. Considerando-se os inse- tos sugadores como principais pragas dos cultivos em nível mundial, as implicações das rela- ções trofobióticas são discutidas no contexto das comunidades de insetos em geral, focalizan- do os problemas que geram em Manejo Integrado de Pragas (MIP), em particular.