Evolutionary Flexibility in Five Hummingbird/Plant Mutualistic Systems: Testing Temporal and Geographic Matching
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Basilinna Genus (Aves: Trochilidae): an Evaluation Based on Molecular Evidence and Implications for the Genus Hylocharis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Revista Mexicana de Biodiversidad 85: 797-807, 2014 DOI: 10.7550/rmb.35769 The Basilinna genus (Aves: Trochilidae): an evaluation based on molecular evidence and implications for the genus Hylocharis El género Basilinna (Aves: Trochilidae): una evaluación basada en evidencia molecular e implicaciones para el género Hylocharis Blanca Estela Hernández-Baños1 , Luz Estela Zamudio-Beltrán1, Luis Enrique Eguiarte-Fruns2, John Klicka3 and Jaime García-Moreno4 1Museo de Zoología, Departamento de Biología Evolutiva, Facultad de Ciencias, Universidad Nacional Autónoma de México. Apartado postal 70- 399, 04510 México, D. F., Mexico. 2Departamento de Ecología Evolutiva, Instituto de Ecología, Universidad Nacional Autónoma de México. Apartado postal 70-275, 04510 México, D. F., Mexico. 3Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA, USA. 4Amphibian Survival Alliance, PO Box 20164, 1000 HD Amsterdam, The Netherlands. [email protected] Abstract. Hummingbirds are one of the most diverse families of birds and the phylogenetic relationships within the group have recently begun to be studied with molecular data. Most of these studies have focused on the higher level classification within the family, and now it is necessary to study the relationships between and within genera using a similar approach. Here, we investigated the taxonomic status of the genus Hylocharis, a member of the Emeralds complex, whose relationships with other genera are unclear; we also investigated the existence of the Basilinna genus. We obtained sequences of mitochondrial (ND2: 537 bp) and nuclear genes (AK-5 intron: 535 bp, and c-mos: 572 bp) for 6 of the 8 currently recognized species and outgroups. -
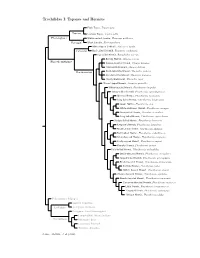
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

Macroscale Analysis of Mistletoe Host Ranges in the Andean‐Patagonian
DR. GUILLERMO AMICO (Orcid ID : 0000-0002-3709-3111) Article type : Research Paper handling Editor: Dr. Diane Byers Macroscale Analysis of Mistletoe Host Ranges in the Andean-Patagonian Forest Article Guillermo C. Amico1*, Daniel L. Nickrent2 and Romina Vidal-Russell1 1 Laboratorio Ecotono, INIBIOMA CONICET (Universidad Nacional del Comahue) Quintral 1250, Bariloche, Río Negro, Argentina 2 Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901-6509 Corresponding author Corresponding author’s e-mail address: [email protected] This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as Accepted doi: 10.1111/plb.12900 This article is protected by copyright. All rights reserved. ABSTRACT The number of host species infected by a mistletoe (host range) is critical in that it influences prevalence, virulence and overall distribution of the parasite; however, macroecological analyses of this life history feature are lacking for many regions. The Andean-Patagonian forest, found along the southern Andes from 35˚S to Tierra del Fuego 55˚S, contains twelve mistletoe species in three families (Loranthaceae, Misodendraceae and Santalaceae). By tabulating herbarium records, the host ranges and geographical distributions of these mistletoes were explored. Our results show that these parasites occur on 43 plant species in 24 families but with varying degrees of specificity. All Misodendrum species and Desmaria mutabilis (Loranthaceae) are specialists that use Nothofagus as their primary hosts. Tristerix and Article Notanthera (Loranthaceae) and Antidaphne and Lepidoceras (Santalaceae) are generalists parasitizing more than six host species from several genera and families. -

Early Evolution of the Angiosperm Clade Asteraceae in the Cretaceous of Antarctica
Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica Viviana D. Barredaa,1,2, Luis Palazzesia,b,1, Maria C. Telleríac, Eduardo B. Oliverod, J. Ian Rainee, and Félix Forestb aDivisión Paleobotánica, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Consejo Nacional de Investigaciones Cientificas y Técnicas, Buenos Aires C1405DJR, Argentina; bJodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom; cLaboratorio de Sistemática y Biología Evolutiva, Museo de La Plata, La Plata B1900FWA, Argentina; dCentro Austral de Investigaciones Científicas, Consejo Nacional de Investigaciones Cientificas y Técnicas, 9410 Ushuaia, Tierra del Fuego, Argentina; and eDepartment of Palaeontology, GNS Science, Lower Hutt 5040, New Zealand Edited by Michael J. Donoghue, Yale University, New Haven, CT, and approved July 15, 2015 (received for review December 10, 2014) The Asteraceae (sunflowers and daisies) are the most diverse Here we report fossil pollen evidence from exposed Campanian/ family of flowering plants. Despite their prominent role in extant Maastrichtian sediments from the Antarctic Peninsula (Fig. 1, Fig. S1, terrestrial ecosystems, the early evolutionary history of this family and SI Materials and Methods, Fossiliferous Localities)(7)thatradi- remains poorly understood. Here we report the discovery of a cally changes our understanding of the early evolution of Asteraceae. number of fossil pollen grains preserved in dinosaur-bearing deposits from the Late Cretaceous of Antarctica that drastically pushes back Results and Discussion the timing of assumed origin of the family. Reliably dated to ∼76–66 The pollen grains reported here and discovered in the Late Cre- Mya, these specimens are about 20 million years older than previ- taceous of Antarctica are tricolporate, microechinate, with long ously known records for the Asteraceae. -

1 AOS Classification Committee – North and Middle America Proposal Set 2020-A 4 September 2019 No. Page Title 01 02 Change Th
AOS Classification Committee – North and Middle America Proposal Set 2020-A 4 September 2019 No. Page Title 01 02 Change the English name of Olive Warbler Peucedramus taeniatus to Ocotero 02 05 Change the generic classification of the Trochilini (part 1) 03 11 Change the generic classification of the Trochilini (part 2) 04 18 Split Garnet-throated Hummingbird Lamprolaima rhami 05 22 Recognize Amazilia alfaroana as a species not of hybrid origin, thus moving it from Appendix 2 to the main list 06 26 Change the linear sequence of species in the genus Dendrortyx 07 28 Make two changes concerning Starnoenas cyanocephala: (a) assign it to the new monotypic subfamily Starnoenadinae, and (b) change the English name to Blue- headed Partridge-Dove 08 32 Recognize Mexican Duck Anas diazi as a species 09 36 Split Royal Tern Thalasseus maximus into two species 10 39 Recognize Great White Heron Ardea occidentalis as a species 11 41 Change the English name of Checker-throated Antwren Epinecrophylla fulviventris to Checker-throated Stipplethroat 12 42 Modify the linear sequence of species in the Phalacrocoracidae 13 49 Modify various linear sequences to reflect new phylogenetic data 1 2020-A-1 N&MA Classification Committee p. 532 Change the English name of Olive Warbler Peucedramus taeniatus to Ocotero Background: “Warbler” is perhaps the most widely used catch-all designation for passerines. Its use as a meaningful taxonomic indicator has been defunct for well over a century, as the “warblers” encompass hundreds of thin-billed, insectivorous passerines across more than a dozen families worldwide. This is not itself an issue, as many other passerine names (flycatcher, tanager, sparrow, etc.) share this common name “polyphyly”, and conventions or modifiers are widely used to designate and separate families that include multiple groups. -

Ecography ECOG-01538 Maglianesi, M
Ecography ECOG-01538 Maglianesi, M. A., Blüthgen, N., Böhning-Gaese, K. and Schleuning, M. 2015. Topographic microclimates drive microhabitat associations at the range margin of a butterfly. – Ecography doi: 10.1111/ecog.01538 Supplementary material Appendix 1 Table A1. List of families, genera and species of plants recorded by identification of pollen loads carried by hummingbird individuals at three elevations in northeastern Costa Rica. Only plant morphotypes that could be identified to species, genus or family level are given. The proportion of pollen identified to species level was 43% and that identified to a higher taxonomic level was 10%; 47% of pollen grains were categorized into pollen morphotypes (not shown here). Plant families are ordered alphabetically within each elevation. Elevation Family Genus Species Low Bromeliaceae Aechmea Aechmea mariareginae Low Acanthaceae Aphelandra Aphelandra storkii Low Bignoniaceae Arrabidaea Arrabidaea verrucosa Low Gesneraciae Besleria Besleria columnoides Low Alstroemeriaceae Bomarea Bomarea obovata Low Gesneriaceae Columnea Columnea linearis Low Gesneraceae Columnea Columnea nicaraguensis Low Gesneraceae Columnea Columnea purpurata Low Gesneraceae Columnea Columnea querceti Low Costaceae Costus Costus pulverulentus Low Costaceae Costus Costus scaber Low Costaceae Costus Costus sp 1 Low Gesneriaceae Drymonia Drymonia macrophylla Low Ericaceae Ericaceae Ericaceae 1 Low Ericaceae Ericaceae Ericaceae 2 Low Bromeliaceae Guzmania Guzmania monostachia Low Rubiaceae Hamelia Hamelia patens Low Heliconiaceae -

Lições Das Interações Planta – Beija-Flor
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA JÉFERSON BUGONI REDES PLANTA-POLINIZADOR NOS TRÓPICOS: LIÇÕES DAS INTERAÇÕES PLANTA – BEIJA-FLOR PLANT-POLLINATOR NETWORKS IN THE TROPICS: LESSONS FROM HUMMINGBIRD-PLANT INTERACTIONS CAMPINAS 2017 JÉFERSON BUGONI REDES PLANTA-POLINIZADOR NOS TRÓPICOS: LIÇÕES DAS INTERAÇÕES PLANTA – BEIJA-FLOR PLANT-POLLINATOR NETWORKS IN THE TROPICS: LESSONS FROM HUMMINGBIRD-PLANT INTERACTIONS Tese apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do Título de Doutor em Ecologia. Thesis presented to the Institute of Biology of the University of Campinas in partial fulfillment of the requirements for the degree of Doctor in Ecology. ESTE ARQUIVO DIGITAL CORRESPONDE À VERSÃO FINAL DA TESE DEFENDIDA PELO ALUNO JÉFERSON BUGONI E ORIENTADA PELA DRA. MARLIES SAZIMA. Orientadora: MARLIES SAZIMA Co-Orientador: BO DALSGAARD CAMPINAS 2017 Campinas, 17 de fevereiro de 2017. COMISSÃO EXAMINADORA Profa. Dra. Marlies Sazima Prof. Dr. Felipe Wanderley Amorim Prof. Dr. Thomas Michael Lewinsohn Profa. Dra. Marina Wolowski Torres Prof. Dr. Vinícius Lourenço Garcia de Brito Os membros da Comissão Examinadora acima assinaram a Ata de Defesa, que se encontra no processo de vida acadêmica do aluno. DEDICATÓRIA À minha família por me ensinar o amor à natureza e a natureza do amor. Ao povo brasileiro por financiar meus estudos desde sempre, fomentando assim meus sonhos. EPÍGRAFE “Understanding patterns in terms of the processes that produce them is the essence of science […]” Levin, S.A. (1992). The problem of pattern and scale in ecology. Ecology 73:1943–1967. AGRADECIMENTOS Manifestar a gratidão às tantas pessoas que fizeram parte direta ou indiretamente do processo que culmina nesta tese não é tarefa trivial. -

Las Asteráceas (Compositae) Del Distrito De Laraos (Yauyos, Lima, Perú)
Revista peruana de biología 23(2): 195 - 220 (2016) Las Asteráceas deISSN-L Laraos, 1561-0837 Yauyos doi: http://dx.doi.org/10.15381/rpb.v23i2.12439 Facultad de Ciencias Biológicas UNMSM TRABAJOS ORIGINALES Las Asteráceas (Compositae) del distrito de Laraos (Yauyos, Lima, Perú) The Asteraceae (Compositae) from Laraos district (Yauyos, Lima, Peru) Hamilton Beltrán Museo de Historia Natural Universidad Nacional Mayor de San Marcos, Av. Arenales 1254 Apartado 14-0434 Lima – Perú Email: [email protected] Resumen El distrito de Laraos registra 155 especies de Asteráceas agrupadas en 66 géneros, 12 tribus y 3 subfamilias. Senecio, Werneria y Baccharis son los géneros con mayor riqueza. Senecio larahuinensis y Conyza coronopi- folia son nuevos registros para la flora del Perú, siendo la primera como especie nueva; además 35 especies se reportan como nuevas para Lima. Se presentan claves dicotómicas para la determinación de las tribus, géneros y especies. Palabras clave: Vertientes occidentales; Asteraceae; endemismo; Perú. Abstract For the district Laraos 155 species of asteraceae grouped into 66 genus, 12 tribes and 3 subfamilies are recorded. Senecio, Baccharis and Werneria are genus more wealth. Senecio larahuinensis and Conyza coro- nopifolia are new records for the flora of Peru as the first new species; further 35 species are new reports for Lima. Dichotomous keys for the identification of tribes, genus and species present. Keywords: Western slopes; Asteraceae; endemic; Peru. Introducción Para Perú, con la publicación del catálogo de plantas con Las asteráceas son la familia de plantas con flores con mayor flores y gimnospermas (Brako & Zarucchi 1993) registraron número de especies, distribuidas en casi toda la superficie terres- 222 géneros y 1432 especies de asteráceas; posteriormente tre, a excepción de los mares y la Antártida, con aproximada- Beltrán y Baldeón (2001) actualizan el registro con 245 gé- mente 1600 géneros y 24000 especies (Bremer 1994, Kadereit neros y 1530 especies. -

Universidad Nacional "San Luis Gonzaga" De Ica Facultad De Farmacia Y Bioquímica
, UNIVERSIDAD NACIONAL "SAN LUIS GONZAGA" DE ICA FACULTAD DE FARMACIA Y BIOQUÍMICA ICA HATUN YACHAY HUASI "CONTENIDO DE POLIFENOLES TOTALES YACTIVIDAD ANTIOXIDANTE IN VITRO DEL EXTRACTO ETANÓLICO DE HOJAS YFLORES DE Chuiraga spinosa Less 11huamanpinta" TESIS: , PARA OPTAR fL TITULO DE: QUÍMICO FARMACÉUTICO PRESENTADO POR: Bach. Sánchez Llamosa Miguel Jesús Bach. Anicaina Pariona Nataly Esther ASESOR: Mg. Juan José A. Palomino Jhong Co-ASESOR: Mg. Osear Herrera Ca~ld'eron ICA- PERÚ 2015 Dedicatoria: A Dios por darnos el conocimiento y estar pendiente de nosotros. A .nuestros padres y hermanos quienes con su amor, apoyo y comprensión incondicional estuvieron siempre a lo largo de nuestra vida estudiantil; a ellos que siempre tuvieron una palabra de aliento en los momentos diffciles y que han sido incentivos de nuestras vidas. A nuestros asesores quienes confiaron en cada una de nosotros para poder llevar acabo el desarrollo de la presente, brindándonos sus conocimientos y apoyo incondicional en esta ardua labor. AGRADECIMIENTOS Nuestro más amplio agradecimiento a los docentes que con su paciencia nos supieron orientar hacia el camino de la superación y el éxito. A nuestros asesores que gracias a su apoyo y dedicación no hubiese sido posible la culminación de la presente investigación Al Jefe del Departamento de Ciencias Químicas por permitirnos usar las instalaciones del laboratorio de Química General Aplicada de la FF.BB - UNICA, para la realización del presente trabajo de tesis. A nuestros profesores de la Facultad de Farmacia y Bioquímica pór todo el conocimiento brindado en estos cinco años de estudios. 3 ÍNDICE Página RESUMEN 7 ABSTRACT 8 INTRODUCCIÓN 9 CAPÍTU~O l. -

Ethnobotanical Study of Medicinal Plants Used by the Andean People of Canta, Lima, Peru
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/266388116 Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru Article in Journal of Ethnopharmacology · June 2007 DOI: 10.1016/j.jep.2006.11.018 CITATIONS READS 38 30 3 authors, including: Percy Amilcar Pollito University of São Paulo 56 PUBLICATIONS 136 CITATIONS SEE PROFILE All content following this page was uploaded by Percy Amilcar Pollito on 14 November 2014. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. Journal of Ethnopharmacology 111 (2007) 284–294 Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru Horacio De-la-Cruz a,∗, Graciela Vilcapoma b, Percy A. Zevallos c a Facultad de Ciencias Biol´ogicas, Universidad Pedro Ruiz Gallo, Lambayeque, Peru b Facultad de Ciencias, Universidad Nacional Agraria La Molina, Lima, Peru c Facultad de Ciencias Forestales, Universidad Nacional Agraria La Molina, Lima, Peru Received 14 June 2006; received in revised form 15 November 2006; accepted 19 November 2006 Available online 2 December 2006 Abstract A survey aiming to document medicinal plant uses was performed in Canta Province Lima Department, in the Peruvians Andes of Peru. Hundred and fifty people were interviewed. Enquiries and informal personal conversations were used to obtain information. Informants were men and women over 30 years old, who work in subsistence agriculture and cattle farming, as well as herbalist. -

The Origin of the Bifurcating Style in Asteraceae (Compositae)
Annals of Botany 117: 1009–1021, 2016 doi:10.1093/aob/mcw033, available online at www.aob.oxfordjournals.org The origin of the bifurcating style in Asteraceae (Compositae) Liliana Katinas1,2,*, Marcelo P. Hernandez 2, Ana M. Arambarri2 and Vicki A. Funk3 1Division Plantas Vasculares, Museo de La Plata, La Plata, Argentina, 2Laboratorio de Morfologıa Comparada de Espermatofitas (LAMCE), Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, La Plata, Argentina and 3Department of Botany, NMNH, Smithsonian Institution, Washington D.C., USA *For correspondence. E-mail [email protected] Received: 20 November 2015 Returned for revision: 22 December 2015 Accepted: 8 January 2016 Published electronically: 20 April 2016 Background and Aims The plant family Asteraceae (Compositae) exhibits remarkable morphological variation in the styles of its members. Lack of studies on the styles of the sister families to Asteraceae, Goodeniaceae and Calyceraceae, obscures our understanding of the origin and evolution of this reproductive feature in these groups. The aim of this work was to perform a comparative study of style morphology and to discuss the relevance of im- portant features in the evolution of Asteraceae and its sister families. Methods The histochemistry, venation and general morphology of the styles of members of Goodeniaceae, Calyceraceae and early branching lineages of Asteraceae were analysed and put in a phylogenetic framework to dis- cuss the relevance of style features in the evolution of these families. Key Results The location of lipophilic substances allowed differentiation of receptive from non-receptive style papillae, and the style venation in Goodeniaceae and Calyceraceae proved to be distinctive. -

Arnaldoa Argentea (Barnadesioideae: Asteraceae), a New Species and a New Generic Record for Ecuador
Arnaldoa argentea (Barnadesioideae: Asteraceae), a New Species and a New Generic Record for Ecuador Carmen Ulloa Ulloa and Peter M. Jùrgensen Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, U.S.A. [email protected]; [email protected] Michael O. Dillon Department of Botany, The Field Museum, Chicago, Illinois 60605, U.S.A. dillon@®eldmuseum.org ABSTRACT. A new species of Asteraceae, Arnal- Erbar & Leins, 2000) on the corollas, achenes, and doa argentea C. Ulloa, P. Jùrgensen & M. O. Dillon, pappus of most species (Bremer, 1994). Most mem- from southern Ecuador is described and illustrated. bers also have peculiar spines in pairs, sometimes It is characterized by its cream-white to light or- solitary or three to ®ve together between the sub- ange corollas and red-brown phyllaries covered by tending leaf and the axillary bud (Bremer, 1994). a dense silvery pubescence, especially on the ad- Various af®nities with other, mainly Andean, genera axial surface. The genus was previously known only of this subfamily have been suggested for Arnaldoa. from northern Peru. A key to the species of Arnal- Morphologically the genus has been placed be- doa is presented. tween Chuquiraga and Dasyphyllum (Cabrera, 1962, 1977), and an intergeneric hybridization be- RESUMEN. Se describe e ilustra una nueva espe- tween Barnadesia and Chuquiraga has been sug- cie de Asteraceae, Arnaldoa argentea C. Ulloa, P. gested (Stuessy et al., 1996). A recent molecular Jùrgensen & M. O. Dillon, del sur de Ecuador. Esta phylogeny (Gustafsson et al., 2001) reveals a well- especie se caracteriza por las corolas de color blan- supported Arnaldoa clade also comprising Fulcaldea co-crema a anaranjado paÂlido y las ®larias cafeÂ- and Dasyphyllum subg.