Neuromodulation of Intrinsic and Synaptic Plasticity in Auditory Cortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

'What' but Not 'Where' Auditory Processing Pathway
NeuroImage 82 (2013) 295–305 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Emotion modulates activity in the ‘what’ but not ‘where’ auditory processing pathway James H. Kryklywy d,e, Ewan A. Macpherson c,f, Steven G. Greening b,e, Derek G.V. Mitchell a,b,d,e,⁎ a Department of Psychiatry, University of Western Ontario, London, Ontario N6A 5A5, Canada b Department of Anatomy & Cell Biology, University of Western Ontario, London, Ontario N6A 5A5, Canada c National Centre for Audiology, University of Western Ontario, London, Ontario N6A 5A5, Canada d Graduate Program in Neuroscience, University of Western Ontario, London, Ontario N6A 5A5, Canada e Brain and Mind Institute, University of Western Ontario, London, Ontario N6A 5A5, Canada f School of Communication Sciences and Disorders, University of Western Ontario, London, Ontario N6A 5A5, Canada article info abstract Article history: Auditory cortices can be separated into dissociable processing pathways similar to those observed in the vi- Accepted 8 May 2013 sual domain. Emotional stimuli elicit enhanced neural activation within sensory cortices when compared to Available online 24 May 2013 neutral stimuli. This effect is particularly notable in the ventral visual stream. Little is known, however, about how emotion interacts with dorsal processing streams, and essentially nothing is known about the impact of Keywords: emotion on auditory stimulus localization. In the current study, we used fMRI in concert with individualized Auditory localization Emotion auditory virtual environments to investigate the effect of emotion during an auditory stimulus localization fi Auditory processing pathways task. Surprisingly, participants were signi cantly slower to localize emotional relative to neutral sounds. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -

Developmental Plasticity of the Glutamate Synapse: Roles of Low Frequency Stimulation, Hebbian Induction and the Nmda Receptor
DEVELOPMENTAL PLASTICITY OF THE GLUTAMATE SYNAPSE: ROLES OF LOW FREQUENCY STIMULATION, HEBBIAN INDUCTION AND THE NMDA RECEPTOR Akademisk avhandling som för avläggande av medicine doktorsexamen vid Sahlgrenska akademin vid Göteborgs universitet kommer att offentligen försvaras i hörsal 2119, Hus 2, Hälsovetarbacken Göteborg, fredagen den 12 februari 2010 kl 09.00 av Joakim Strandberg Fakultetsopponent: Professor Martin Garwicz Institutionen för experimentell medicinsk vetenskap Lunds universitet Avhandlingen baseras på följande delarbeten: I. Strandberg J., Wasling P. and Gustafsson B. Modulation of low frequency induced synaptic depression in the developing CA3-CA1 hippocampal synapses by NMDA and metabotropic glutamate receptor activation. Journal of Neurophysiology (2009) 101:2252-2262 II. Strandberg J. and Gustafsson B. Lasting activity-induced depression of previously non-stimulated CA3-CA1 synapses in the developing hippocampus; critical and complex role of NMDA receptors. In manuscript III. Strandberg J. and Gustafsson B. Hebbian activity does not stabilize synaptic transmission at CA3-CA1 synapses in the developing hippocampus. In manuscript Göteborg 2010 DEVELOPMENTAL PLASTICITY OF THE GLUTAMATE SYNAPSE: ROLES OF LOW FREQUENCY STIMULATION, HEBBIAN INDUCTION AND THE NMDA RECEPTOR Joakim Strandberg Department of Physiology, Institute of Neuroscience and Physiology, Univeristy of Gothenburg, Sweden, 2010 Abstract The glutamate synapse is by far the most common synapse in the brain and acts via postsynaptic AMPA, NMDA and mGlu receptors. During brain development there is a continuous production of these synapses where those partaking in activity resulting in neuronal activity are subsequently selected to establish an appropriate functional pattern of synaptic connectivity while those that do not are elimimated. Activity dependent synaptic plasticities, such as Hebbian induced long-term potentiation (LTP) and low frequency (1 Hz) induced long-term depression (LTD) have been considered to be of critical importance for this selection. -

Neuronal Organization in the Inferior Colliculus Revisited with Cell-Type- Dependent Monosynaptic Tracing
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Neuronal organization in the inferior colliculus revisited with cell-type- dependent monosynaptic tracing Chenggang Chen1, Mingxiu Cheng1,2, Tetsufumi Ito3 and Sen Song1 1Tsinghua Laboratory of Brain and Intelligence (THBI) and Department of Biomedical Engineering, Beijing Innovation Center for Future Chip, Center for Brain-Inspired Computing Research, McGovern Institute for Brain Research, Tsinghua University, Beijing, 100084, China 2National Institute of Biological Sciences, Beijing, 102206, China 3Anatomy II, School of Medicine, Kanazawa Medical University, Uchinada, Ishikawa, 920-0293, Japan DOI: 10.1523/JNEUROSCI.2173-17.2018 Received: 31 July 2017 Revised: 2 February 2018 Accepted: 7 February 2018 Published: 24 February 2018 Author contributions: C.C., T.I., and S.S. designed research; C.C. and M.C. performed research; C.C. and T.I. analyzed data; C.C. wrote the first draft of the paper; C.C., T.I., and S.S. edited the paper; C.C., T.I., and S.S. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. This work was supported by funding from the National Natural Science Foundation of China (31571095, 91332122, for S.S.), Special Fund of Suzhou-Tsinghua Innovation Leading Action (for S.S.), Beijing Program on the Study of Brain-Inspired Computing System and Related Core Technologies (for S.S.), Beijing Innovation Center for Future Chip (for S.S.), and Chinese Academy of Sciences Institute of Psychology Key Laboratory of Mental Health Open Research Grant (KLMH2012K02, for S.S.), grants from Ministry of Education, Science, and Culture of Japan (KAKENHI grant, Grant numbers 16K07026 and 16H01501; for T.I.), and Takahashi Industrial and Economic Research Foundation (for T.I.). -
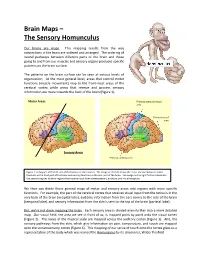
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Subdivisions of Auditory Cortex and Processing Streams in Primates
Colloquium Subdivisions of auditory cortex and processing streams in primates Jon H. Kaas*† and Troy A. Hackett‡ Departments of †Psychology and ‡Hearing and Speech Sciences, Vanderbilt University, Nashville, TN 37240 The auditory system of monkeys includes a large number of histochemical studies in chimpanzees and humans, and nonin- interconnected subcortical nuclei and cortical areas. At subcortical vasive functional studies in humans. levels, the structural components of the auditory system of mon- keys resemble those of nonprimates, but the organization at The Core Areas of Auditory Cortex cortical levels is different. In monkeys, the ventral nucleus of the Originally, auditory cortex of monkeys was thought to be orga- medial geniculate complex projects in parallel to a core of three nized much as in cats, with a single primary area, AI, in the primary-like auditory areas, AI, R, and RT, constituting the first cortex of the lower bank of the lateral sulcus and a second area, stage of cortical processing. These areas interconnect and project AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with to the homotopic and other locations in the opposite cerebral the early view that auditory, somatosensory, and visual systems hemisphere and to a surrounding array of eight proposed belt all have two fields. However, we now know that primates have areas as a second stage of cortical processing. The belt areas in turn a number of sensory representations for each modality, and project in overlapping patterns to a lateral parabelt region with at several somatosensory and auditory fields can be considered least rostral and caudal subdivisions as a third stage of cortical primary or primary like in character. -

DR. Sanaa Alshaarawy
By DR. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal, mid “Trigeminal level” and rostral). 3. Mid brain ( superior and inferior colliculi). Describe the Reticular formation (structure, function and pathway) being an important content of the brain stem. 2 1. Traversed by the Central Canal. Motor Decussation*. Spinal Nucleus of Trigeminal (Trigeminal sensory nucleus)* : ➢ It is a larger sensory T.S of Caudal part of M.O. nucleus. ➢ It is the brain stem continuation of the Substantia Gelatinosa of spinal cord 3 The Nucleus Extends : Through the whole length of the brain stem and upper segments of spinal cord. It lies in all levels of M.O, medial to the spinal tract of the trigeminal. It receives pain and temperature from face, forehead. Its tract present in all levels of M.O. is formed of descending fibers that terminate in the trigeminal nucleus. 4 It is Motor Decussation. Formed by pyramidal fibers, (75-90%) cross to the opposite side They descend in the Decuss- = crossing lateral white column of the spinal cord as the lateral corticospinal tract. The uncrossed fibers form the ventral corticospinal tract. 5 Traversed by Central Canal. Larger size Gracile & Cuneate nuclei, concerned with proprioceptive deep sensations of the body. Axons of Gracile & Cuneate nuclei form the internal arcuate fibers; decussating forming Sensory Decussation. Pyramids are prominent ventrally. 6 Formed by the crossed internal arcuate fibers Medial Leminiscus: Composed of the ascending internal arcuate fibers after their crossing. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 18, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

Specific Involvement of Postsynaptic Glun2b- Containing NMDA
Specific involvement of postsynaptic GluN2B- containing NMDA receptors in the developmental elimination of corticospinal synapses Takae Ohnoa, Hitoshi Maedaa, Naoyuki Murabea, Tsutomu Kamiyamaa, Noboru Yoshiokaa, Masayoshi Mishinab, and Masaki Sakuraia,1 aDepartment of Physiology, School of Medicine, Teikyo University, Tokyo 173-8605, Japan; and bDepartment of Molecular Neurobiology and Pharmacology, Graduate School of Medicine, University of Tokyo, Tokyo 113-8655, Japan Edited* by Masao Ito, RIKEN Brain Science Institute, Wako, Japan, and approved July 19, 2010 (received for review July 15, 2009) The GluN2B (GluRε2/NR2B) and GluN2A (GluRε1/NR2A) NMDA re- spinal gray matter at 7 d in vitro (DIV) but the synapses on the ceptor (NMDAR) subtypes have been differentially implicated in ventral side were subsequently eliminated through a process that activity-dependent synaptic plasticity. However, little is known was blocked by an NMDAR antagonist (22, 23). This type of about the respective contributions made by these two subtypes synapse elimination was also seen in vivo in the rat and followed to developmental plasticity, in part because studies of GluN2B KO a time course similar to that seen in vitro (24), and similar − − − − [Grin2b / (2b / )] mice are hampered by early neonatal mortality. elimination of synapses from ventral areas of the SpC during We previously used in vitro slice cocultures of rodent cerebral development has also been observed in cats (reviewed in ref. 25). cortex (Cx) and spinal cord (SpC) to show that corticospinal (CS) Those findings, together with the observation that the major synapses, once present throughout the SpC, are eliminated from NMDAR subunit mediating CS excitatory postsynaptic currents the ventral side during development in an NMDAR-dependent (EPSCs) appears to shift from 2B to 2A early during development manner. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts.