Species Status Assessment for San Clemente Island Lotus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Combination in Acmispon (Fabaceae: Loteae) for California Luc Brouillet Université De Montréal, Montreal, Canada
Aliso: A Journal of Systematic and Evolutionary Botany Volume 28 | Issue 1 Article 6 2010 A New Combination in Acmispon (Fabaceae: Loteae) for California Luc Brouillet Université de Montréal, Montreal, Canada Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Brouillet, Luc (2010) "A New Combination in Acmispon (Fabaceae: Loteae) for California," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 28: Iss. 1, Article 6. Available at: http://scholarship.claremont.edu/aliso/vol28/iss1/6 Aliso, 28, p. 63 ’ 2010, Rancho Santa Ana Botanic Garden A NEW COMBINATION IN ACMISPON (FABACEAE: LOTEAE) FOR CALIFORNIA LUC BROUILLET Herbier Marie-Victorin, Institut de recherche en biologie ve´ge´tale, Universite´de Montre´al, 4101 Sherbrooke St. E, Montreal, Quebec, Canada H1X 2B2 ([email protected]) ABSTRACT The new combination Acmispon argophyllus (A.Gray) Brouillet var. niveus (Greene) Brouillet is made. Key words: Acmispon, California, Fabaceae, Loteae, North America, Santa Cruz Island. Acmispon argophyllus (A.Gray) Brouillet var. niveus (Greene) Variety niveus is a northern Channel Islands (California) Brouillet, comb. et stat. nov.—TYPE: California. Santa endemic that is distinguished from the closely related southern Cruz Island [s.d.], E.L. Greene s.n. (holotype CAS!, isotype Channel Islands endemic var. adsurgens (Dunkle) Brouillet by (part of type) UC!). stems ascending to erect (vs. erect), less crowded leaves, a silky (vs. silvery) indumentum, smaller umbels (6–10 vs. 10–13 Basionym: Syrmatium niveum Greene, Bull. Calif. Acad. Sci. 2: 148 flowers), and slightly longer calyx lobes (2.5–5.0 vs. -

Legally Listed Species of the California Central Coast Region (U S Fish and Wildlife Service and /Or the State of California)
Legally Listed Species of the California Central Coast Region (U S Fish and Wildlife Service and /or the State of California) (Monterey, San Benito, San Luis Obispo, western Kern, Santa Barbara, and Ventura counties) The following taxa, in alphabetical order by scientific name, are listed either by the U. S. Fish and Wildlife Service (Endangered Species Act) or by the State of California, Department of Fish and Wildlife, Natural Diversity Database. A comprehensive list for the State of California is updated quarterly by the California Natural Diversity Database. [Special Vascular Plants, Bryophytes, and Lichens List.] The distribution of these species has been documented for California’s central coast region from Monterey and San Benito counties south to Ventura County, and including western Kern County. Scientific names are those used in Baldwin et. al., 2012, The Jepson Manual: vascular plants of California, UC Press, Berkeley. Where nomenclature has changed from the name used initially in the listing process, they are referenced to the current name (e.g., Arabis hoffmannii = Boechera hoffmannii). Listing Status FE = federally endangered (Endangered Species Act, 1973 as amended) FT = federally threatened (Endangered Species Act, 1973 as amended) SE = state endangered (California Endangered Species Act; Fish and Game Code §2050 et seq.) ST = state threatened (California Endangered Species Act; Fish and Game Code §2050 et seq.) SR = state rare (California Native Plant Protection Act; Fish and Game Code §1900 et seq.). Scientific Name Common Name Status Acmispon argophyllus var. niveus Santa Cruz Island birds-foot trefoil SE Arabis hoffmannii = Boechera hoffmannii Boechera hoffmannii Hoffmann’s rock-cress FE Arctostaphylos confertiflora Santa Rosa Island manzanita FE Arctostaphylos hookeri subsp. -

Lotus Scoparius (Nutt. in Torr. & A. Gray) Ottley [= Acmispon Glaber
SPECIES Lotus scoparius (Nutt. in Torr. & A. Gray) Ottley [= Acmispon glaber (Vogel) Brouillet] NRCS CODE: Tribe: Loteae LOSC2 Subfamily: Papilionoideae LOSCS2 Family: Fabaceae LOSCB Order: Fabales Subclass: Rosidae Class: Magnoliopsida LOSCB , Riverside Co., A. Montalvo 2009 LOSCS2, Monterey coast, A. Montalvo 2003 LOSCB, Riverside Co., A. Montalvo 2010, Subspecific taxa 1. LOSCS2 1. Lotus scoparius var. scoparius 2. LOSCB 2. Lotus scoparius (Nutt.) Ottley var. brevialatus Ottley Synonyms 1. Acmispon glaber (Vogel) Brouillet var. glaber [New name in Jepson Manual 2nd Edition, JepsonOnline 2010] Hosackia scoparia Nutt. in T. and G. (taxa numbered as above) H. glaber Greene H. crassifolia Nutt., not Benth L. glaber Greene, not Mill. L. scoparius (Torr. & A. Gray) Ottley L. scoparius (Nutt. in T. & G.) Ottley ssp. scoparius (Ottley) Munz Lotus scoparius (Nutt.) Ottley var. perplexans Hoover p.p. Syrmatium glabrum Vogel 2. Acmispon glaber (Vogel) Brouillet var. brevialatus (Ottley) Brouillet [New name in Jepson Manual 2nd Edition] Hosackia glabra (Vogel) Torr. var. brevialata (Ottley) Abrams Lotus scoparius (Torr. & A. Gray) Ottley var. brevialatus Ottley Lotus scoparius (Nutt. in T. & G.) Ottley ssp. brevialatus (Ottley) Munz Common name General for species: California broom, deerweed 1. coastal deerweed, common deerweed (taxa numbered as above) 2. desert deerweed, western bird's foot trefoil, short-winged deerweed (Roberts 2008, Painter 2009, USDA PLANTS 2010). Over 45 taxa of Lotus were recognized in Isely's treatment in Hickman (1993) for California. These taxa had been grouped and regrouped into various species as well as subgenera or genera based on morphology for over a century. Allan & Porter (2000) analyzed DNA (ITS and nuclear ribosomal DNA), geographic, and morphological data for more than 45 taxa of Lotus together with additional related taxa of Loteae and found several geographically distinct lineages. -
![Acmispon Glaber (Vogel) Brouillet [Updated 2017] = Lotus Scoparius (Nutt](https://docslib.b-cdn.net/cover/3383/acmispon-glaber-vogel-brouillet-updated-2017-lotus-scoparius-nutt-1833383.webp)
Acmispon Glaber (Vogel) Brouillet [Updated 2017] = Lotus Scoparius (Nutt
I. SPECIES Acmispon glaber (Vogel) Brouillet [Updated 2017] = Lotus scoparius (Nutt. in Torr. & A. Gray) Ottley NRCS CODE: [none for Tribe: Loteae Family: Fabaceae Subclass: Rosidae Acmispon] Subfamily: Papilionoideae Order: Fabales Class: Magnoliopsida [LOSC2 code for L. 4 mm scoparius ] Acmispon glaber var. brevialatus [= Lotus scoparius var. brevialatus] 15 mm seedling with linear cotyledons and first pair of true leaves 4 mm Acmispon glaber var. glaber erect form prostrate form on Monterey coast A. Subspecific taxa 1. no NRCS code 1. Acmispon glaber (Vogel) Brouillet var. glaber 2. no NRCS code 2. Acmispon glaber (Vogel) Brouillet var. brevialatus (Ottley) Brouillet [accepted by Baldwin et al. (2012), Jepson eFlora (2017)] B. Common name General for species: deerweed, California broom 1. coastal deerweed, common deerweed, deerweed, coastal deerbroom (taxa numbered as above; 2. short-winged deerweed, desert deerweed, desert deerbroom, western bird's foot trefoil names listed first used below) (Roberts 2008, Allen & Roberts 2013, Calflora 2016, USDA PLANTS 2016). Last modified: 10/19/2018 LOSC2 Update, 1 Printed: 10/19/2018 C. Synonyms 1. LOSCS2 1. Lotus scoparius (Nutt.) Ottley var. scoparius Hosackia scoparia Nutt. in T. and G. H. glaber Greene H. crassifolia Nutt., not Benth L. glaber Greene, not Mill. L. scoparius (Torr. & A. Gray) Ottley L. scoparius (Nutt. in T. & G.) Ottley ssp. scoparius (Ottley) Munz Lotus scoparius (Nutt.) Ottley var. perplexans Hoover p.p. Syrmatium glabrum Vogel 2. LOSCB 2. Lotus scoparius (Nutt.) Ottley var. brevialatus Ottley (taxa numbered as above) Hosackia glabra (Vogel) Torr. var. brevialata (Ottley) Abrams Lotus scoparius (Torr. & A. Gray) Ottley var. brevialatus Ottley Lotus scoparius (Nutt. -
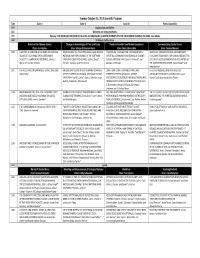
Tuesday October 23, 2012 Scientific Program
Tuesday October 23, 2012 Scientific Program Time Salon I Salon II Salon III Puerto Escondido 0800 Registration and Coffee 0845 Welcome and Announcements 0900 Plenary: THE GEOLOGIC RECORD OF GLACIAL‐INTERGLACIAL CLIMATE CHANGES ON THE CALIFORNIA CHANNEL ISLANDS‐ Dan Muhs 1000 30 Minute Coffee Break History of the Channel Islands Changes in Assemblages of Flora and Fauna Trends in Intertidal and Marine Ecosystems Communicating Coastal Issues Chair: Ann Huston Chair: Stacey Ostermann‐Kelm Chair: Mary Elaine Helix Chair: Yvonne Menard 1030 A HISTORY OF AVIATION IN THE NORTHERN CHANNEL DOCUMENTING THE PALEONTOLOGICAL RESOURCES OF TIDEPOOLING THROUGH TIME: 30 YEARS OF ROCKY SANCTUARY ADVISORY COUNCIL AND SANCTUARY ISLANDS OF CALIFORNIA: FROM GENTLEMEN'S NATIONAL PARK SERVICE AREAS OF THE SOUTHERN INTERTIDAL COMMUNITY MONITORING AT CHANNEL EDUCATION TEAM MODEL FOR COMMUNICATING THE NOVELTY TO COMMERCIAL ENTERPRISE. Charles J CALIFORNIA COAST AND ISLANDS. Justin S. Tweet*, ISLANDS NATIONAL PARK. Daniel V. Richards* and EFFECTS OF OCEAN ACIDIFICATION IN THE WATERS OF Rennie, III* and Ian Williams Vincent L. Santucci, and Tim Connors Stephen G. Whitaker THE SANTA BARBARA CHANNEL. Maria Petueli* and Amanda Allen 1045 THE MILLS YEARS ON SAN MIGUEL ISLAND, 1869‐1888. AN 8000 YEAR RECORD OF BIRD REMAINS FROM CAVE LONG‐TERM CLIMATE VARIABILITY AND LINKS EFFECTIVE MESSAGES AND STRATIGIES FOR Marla Daily* OF THE CHIMNEYS (CA‐SMI‐603), SAN MIGUEL ISLAND, BETWEEN PATTERNS OF COASTAL MARINE COMMUNICATING OCEAN ACIDIFICATION. Laura CALIFORNIA. Paul W. Collins*, Emily L. Whistler, Daniel INVERTEBRATE RECRUITMENT AND ADULT ABUNDANCE Francis*, Julie Bursek and Claire Fackler Guthrie, and René L. Vellanoweth AROUND SANTA CRUZ ISLAND FROM 1995‐2010. -

Checklist of the Vascular Plants of San Diego County 5Th Edition
cHeckliSt of tHe vaScUlaR PlaNtS of SaN DieGo coUNty 5th edition Pinus torreyana subsp. torreyana Downingia concolor var. brevior Thermopsis californica var. semota Pogogyne abramsii Hulsea californica Cylindropuntia fosbergii Dudleya brevifolia Chorizanthe orcuttiana Astragalus deanei by Jon P. Rebman and Michael G. Simpson San Diego Natural History Museum and San Diego State University examples of checklist taxa: SPecieS SPecieS iNfRaSPecieS iNfRaSPecieS NaMe aUtHoR RaNk & NaMe aUtHoR Eriodictyon trichocalyx A. Heller var. lanatum (Brand) Jepson {SD 135251} [E. t. subsp. l. (Brand) Munz] Hairy yerba Santa SyNoNyM SyMBol foR NoN-NATIVE, NATURaliZeD PlaNt *Erodium cicutarium (L.) Aiton {SD 122398} red-Stem Filaree/StorkSbill HeRBaRiUM SPeciMeN coMMoN DocUMeNTATION NaMe SyMBol foR PlaNt Not liSteD iN THE JEPSON MANUAL †Rhus aromatica Aiton var. simplicifolia (Greene) Conquist {SD 118139} Single-leaF SkunkbruSH SyMBol foR StRict eNDeMic TO SaN DieGo coUNty §§Dudleya brevifolia (Moran) Moran {SD 130030} SHort-leaF dudleya [D. blochmaniae (Eastw.) Moran subsp. brevifolia Moran] 1B.1 S1.1 G2t1 ce SyMBol foR NeaR eNDeMic TO SaN DieGo coUNty §Nolina interrata Gentry {SD 79876} deHeSa nolina 1B.1 S2 G2 ce eNviRoNMeNTAL liStiNG SyMBol foR MiSiDeNtifieD PlaNt, Not occURRiNG iN coUNty (Note: this symbol used in appendix 1 only.) ?Cirsium brevistylum Cronq. indian tHiStle i checklist of the vascular plants of san Diego county 5th edition by Jon p. rebman and Michael g. simpson san Diego natural history Museum and san Diego state university publication of: san Diego natural history Museum san Diego, california ii Copyright © 2014 by Jon P. Rebman and Michael G. Simpson Fifth edition 2014. isBn 0-918969-08-5 Copyright © 2006 by Jon P. -
![Acmispon Glaber (Vogel) Brouillet [Updated 2017] = Lotus Scoparius (Nutt](https://docslib.b-cdn.net/cover/9168/acmispon-glaber-vogel-brouillet-updated-2017-lotus-scoparius-nutt-4379168.webp)
Acmispon Glaber (Vogel) Brouillet [Updated 2017] = Lotus Scoparius (Nutt
I. SPECIES Acmispon glaber (Vogel) Brouillet [Updated 2017] = Lotus scoparius (Nutt. in Torr. & A. Gray) Ottley NRCS CODE: [none for Tribe: Loteae Family: Fabaceae Subclass: Rosidae Acmispon] Subfamily: Papilionoideae Order: Fabales Class: Magnoliopsida [LOSC2 code for L. 4 mm scoparius ] Acmispon glaber var. brevialatus [= Lotus scoparius var. brevialatus] 15 mm seedling with linear cotyledons and first pair of true leaves 4 mm Acmispon glaber var. glaber erect form prostrate form on Monterey coast A. Subspecific taxa 1. no NRCS code 1. Acmispon glaber (Vogel) Brouillet var. glaber 2. no NRCS code 2. Acmispon glaber (Vogel) Brouillet var. brevialatus (Ottley) Brouillet [accepted by Baldwin et al. (2012), Jepson eFlora (2017)] B. Common name General for species: deerweed, California broom 1. coastal deerweed, common deerweed, deerweed, coastal deerbroom (taxa numbered as above; 2. short-winged deerweed, desert deerweed, desert deerbroom, western bird's foot trefoil names listed first used below) (Roberts 2008, Allen & Roberts 2013, Calflora 2016, USDA PLANTS 2016). Last modified: 10/19/2018 LOSC2 Update, 1 Printed: 10/19/2018 C. Synonyms 1. LOSCS2 1. Lotus scoparius (Nutt.) Ottley var. scoparius Hosackia scoparia Nutt. in T. and G. H. glaber Greene H. crassifolia Nutt., not Benth L. glaber Greene, not Mill. L. scoparius (Torr. & A. Gray) Ottley L. scoparius (Nutt. in T. & G.) Ottley ssp. scoparius (Ottley) Munz Lotus scoparius (Nutt.) Ottley var. perplexans Hoover p.p. Syrmatium glabrum Vogel 2. LOSCB 2. Lotus scoparius (Nutt.) Ottley var. brevialatus Ottley (taxa numbered as above) Hosackia glabra (Vogel) Torr. var. brevialata (Ottley) Abrams Lotus scoparius (Torr. & A. Gray) Ottley var. brevialatus Ottley Lotus scoparius (Nutt. -
Plant Carnivory and the Evolution of Novelty in Sarracenia Alata
Plant Carnivory and the Evolution of Novelty in Sarracenia alata Dissertation Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Graduate School of the Ohio State University By Gregory Lawrence Wheeler, MS Graduate Program in Evolution, Ecology, and Organismal Biology The Ohio State University 2018 Dissertation Committee: Dr. Bryan C. Carstens, Adviser Dr. Marymegan Daly Dr. Zakee Sabree Dr. Andrea Wolfe Copyright by Gregory Lawrence Wheeler 2018 Abstract Most broadly, this study aimed to develop a better understanding of how organisms evolve novel functions and traits, and examine how seemingly complex adaptive trait syndromes can convergently evolve. As an ideal example of this, the carnivorous plants were chosen. This polyphyletic grouping contains taxa derived from multiple independent evolutionary origins, in at least five plant orders, and has resulted in striking convergence of niche and morphology. First, a database study was performed, with the goal of understanding the evolutionary trends that impact carnivorous plants as a whole. Using carnivorous and non-carnivorous plant genomes available from GenBank. An a priori list of Gene Ontology-coded functions implicated in plant carnivory by earlier studies was constructed via literature review. Experimental and control samples were tested for statistical overrepresentation of these functions. It was found that, while some functions were significant in some taxa, there was no overall shared signal of plant carnivory, with each taxon presumably having selected for a different subset of these functions. Next, analyses were performed that targeted Sarracenia alata specifically. A reference genome for S. alata was assembled using PacBio, Illumina, and BioNano data and annotated using MAKER-P with additional preliminary database filtration. -

Statement of Qualifications
Statement of Qualifications March 2020 Contact: Ken Owen Elihu Gevirtz Executive Director Senior Ecologist [email protected] • (805) 448-5726 [email protected] • (805) 448-4175 928 Carpinteria St. Ste. #3, Santa Barbara, CA 93103 • www.cirweb.org 501c3 Tax ID #61-1463876 • Contractor’s License #1056865 Channel Islands Restoration Statement of Qualifications EXPERIENCE Channel Islands Restoration (CIR) has been restoring habitats on the Channel Islands and the coastal areas of Southern California since 2001. We specialize in eradicating non-native invasive species, propagating native plants, planting native plants, installing irrigation systems, preparing habitat restoration plans and conducting botanical and biological surveys. CIR personnel have expertise in the identification of native and non-native plants, and threatened and endangered species. CIR is a licensed landscape contractor and licensed to apply herbicides. CIR has worked on 30 different projects on the Channel Islands, and more than 70 projects in Santa Barbara and Ventura Counties. Our clients include four federal agencies, six state agencies, nine local or tribal agencies and sixteen private entities. Mainland Projects Santa Barbara County Andree Clark Bird Refuge/Santa Barbara Zoo invasive weed eradication and re-vegetation Arroyo Burro Creek Arundo eradication Arroyo Hondo Preserve Invasive weed eradication Arroyo Hondo Preserve Construction monitoring to protect Steelhead and CA Red-Legged Frog Atascadero Creek Invasive weed eradication and re-vegetation Bixby Ranch Botanical -
Rancho Santa Ana Botanic Garden Occasional Publications
¬ RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS NUMBER 16 A CONSERVATION ASSESSMENT FOR ACMISPON DENDROIDEUS VAR. TRASKIAE (SAN CLEMENTE ISLAND LOTUS, FABACEAE) SULA VANDERPLANK, KIMBERLY O’CONNOR, BRYAN MUNSON AND DAWN LAWSON Published by Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711 2018 RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS NUMBER 16 A CONSERVATION ASSESSMENT FOR ACMISPON DENDROIDEUS VAR. TRASKIAE (SAN CLEMENTE ISLAND LOTUS, FABACEAE) SULA VANDERPLANK, KIMBERLY O’CONNOR, BRYAN MUNSON AND DAWN LAWSON RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS (ISSN 1094-1398) are published at irregular intervals in volumes of various sizes. This series of publications is designed to feature results of original botanical research by members of the Rancho Santa Ana Botanic Garden staff, or by botanists who have collaborated in a Garden program. Proceedings of symposia sponsored by the Garden may also be published in this series. The RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS series is published by Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711-3157. For information about orders for RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS, contact Irene Holiman at the address above or via e-mail: [email protected] or fax at (909) 626-7670. For all other inquiries, contact Vanessa Ashworth at [email protected]. General information about the Garden and its programs can be obtained at www.rsabg.org. (Cover photograph by Sula Vanderplank) PUBLICATION DATA A Conservation Assessment for Acmispon dendroideus var. traskiae (San Clemente Island Lotus, Fabaceae). September 2018. Sula Vanderplank, Kimberly O’Connor, Bryan Munson and Dawn Lawson. -
Pine Mountain Ridge, Ventura County, California by David L
Vascular Plants of Pine Mountain Ridge, Ventura County, California By David L. Magney Botanical Name Common Name Habit Family Abies concolor California White Fir T Pinaceae Acanthoscyphus parishii var. abramsii Abrams Spineflower AH Polygonaceae Acanthoscyphus parishii var. parishii Parish's Oxytheca AH Polygonaceae Achillea millefolium var. occidentale Woolly White Yarrow PH Asteraceae Achillea millefolium var. pacifica Pacific Woolly White Yarrow PH Asteraceae Achyrachaena mollis Blow Wives AH Asteraceae Acmispon americanus var. americanus Spanish Clover AH Fabaceae Acmispon argophyllus ssp. argophyllus Silver Deervetch PH Fabaceae Acmispon brachycarpus Hill Lotus AH Fabaceae Acmispon glaber var. glaber Deerweed S Fabaceae Acmispon grandiflorus var. grandiflorus Large-flowered Lotus PH Fabaceae Acmispon nevadensis var. davidsonii Sulphur-flowered Lotus PH Fabaceae Acmispon oblongifolia var. oblongifolia Narrow-leaved Hosackia PH Fabaceae Acmispon parviflorus Tiny Lotus AH Fabaceae Acmispon strigosus var. strigosus Strigose Lotus AH Fabaceae Acmispon wrangelianus Chile Lotus AH Fabaceae Acourtia microcephala Sacapellote PH Asteraceae Adenostoma fasciculatum var. fasciculatum Chamise S Rosaceae Agoseris grandiflora Bigflower Mountain Dandelion AH Asteraceae Agoseris heterophylla var. heterophylla Annual Mountain Dandelion AH Asteraceae Agoseris retrorsa Mountain Dandelion AH Asteraceae Agrostis exarata Western Spike Bentgrass PG Poaceae Allium burlewii Burlew Onion PG Alliaceae Allium diabloense Diablo Onion PG Alliaceae Allium fimbriatum -

Chew's Ridge, Carmel Valley
CALIFORNIA NATIVE PLANT SOCIETY – VASCULAR PLANTS CHEWS RIDGE Acer macrophyllum - big-leaved maple Calochortus splendens - lilac mariposa Acmispon americanus var. americanus - Spanish clover Calycadenia truncata - rosin weed Acmispon argophyllus - silver-leaved lotus Calyptridium monandrum - common calyptridium Acmispon brachycarpus - short-podded lotus Calyptridium parryi var. hesseae - Santa Cruz mountains Acmispon glaber - deerweed pussypaws Acmispon grandiflorus - large-flowered lotus Calystegia malacophylla ssp. pedicellata - woolly morning-glory Acmispon parviflorus - small-flowered lotus Camissonia strigulosa - contorted primrose Acmispon strigosus - Bishop's lotus Camissoniopsis hirtella - small primrose Acmispon wrangelianus - Chile lotus Camissoniopsis ignota - primrose Adenostoma fasciculatum - chamise Camissoniopsis luciae - primrose Agoseris grandiflora - large-flowered agoseris Camissoniopsis micrantha - small primrose Agoseris heterophylla - annual dandelion Capsella bursa-pastoris - shepherd's purse Agoseris retrorsa - spear-leaved agoseris Carex alma - sturdy sedge Agrostis exarata - western bent-grass Carex bolanderi - Bolander's sedge Aira caryophyllea - silvery hair-grass Carex globosa - round-fruited sedge Allium campanulatum - sierra onion Carex multicaulis - many stemmed sedge Allophyllum divaricatum - straggling gilia Carex serratodens - bifid sedge Allophyllum gilioides - straggling gilia Carex subfusca - rusty sedge Alnus rhombifolia - white alder Castilleja applegatei ssp. martinii - Martin's paint-brush Amelanchier