Population-Level Genetic Structure of Acmispon Argophyllus on the Channel Islands of California
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vascular Flora of the Upper Santa Ana River Watershed, San Bernardino Mountains, California
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/281748553 THE VASCULAR FLORA OF THE UPPER SANTA ANA RIVER WATERSHED, SAN BERNARDINO MOUNTAINS, CALIFORNIA Article · January 2013 CITATIONS READS 0 28 6 authors, including: Naomi S. Fraga Thomas Stoughton Rancho Santa Ana B… Plymouth State Univ… 8 PUBLICATIONS 14 3 PUBLICATIONS 0 CITATIONS CITATIONS SEE PROFILE SEE PROFILE Available from: Thomas Stoughton Retrieved on: 24 November 2016 Crossosoma 37(1&2), 2011 9 THE VASCULAR FLORA OF THE UPPER SANTA ANA RIVER WATERSHED, SAN BERNARDINO MOUNTAINS, CALIFORNIA Naomi S. Fraga, LeRoy Gross, Duncan Bell, Orlando Mistretta, Justin Wood1, and Tommy Stoughton Rancho Santa Ana Botanic Garden 1500 North College Avenue Claremont, California 91711 1Aspen Environmental Group, 201 North First Avenue, Suite 102, Upland, California 91786 [email protected] All Photos by Naomi S. Fraga ABSTRACT: We present an annotated catalogue of the vascular flora of the upper Santa Ana River watershed, in the southern San Bernardino Mountains, in southern California. The catalogue is based on a floristic study, undertaken from 2008 to 2010. Approximately 65 team days were spent in the field and over 5,000 collections were made over the course of the study. The study area is ca. 155 km2 in area (40,000 ac) and ranges in elevation from 1402 m to 3033 m. The study area is botanically diverse with more than 750 taxa documented, including 56 taxa of conservation concern and 81 non-native taxa. Vegetation and habitat types in the area include chaparral, evergreen oak forest and woodland, riparian forest, coniferous forest, montane meadow, and pebble plain habitats. -

A New Combination in Acmispon (Fabaceae: Loteae) for California Luc Brouillet Université De Montréal, Montreal, Canada
Aliso: A Journal of Systematic and Evolutionary Botany Volume 28 | Issue 1 Article 6 2010 A New Combination in Acmispon (Fabaceae: Loteae) for California Luc Brouillet Université de Montréal, Montreal, Canada Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Brouillet, Luc (2010) "A New Combination in Acmispon (Fabaceae: Loteae) for California," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 28: Iss. 1, Article 6. Available at: http://scholarship.claremont.edu/aliso/vol28/iss1/6 Aliso, 28, p. 63 ’ 2010, Rancho Santa Ana Botanic Garden A NEW COMBINATION IN ACMISPON (FABACEAE: LOTEAE) FOR CALIFORNIA LUC BROUILLET Herbier Marie-Victorin, Institut de recherche en biologie ve´ge´tale, Universite´de Montre´al, 4101 Sherbrooke St. E, Montreal, Quebec, Canada H1X 2B2 ([email protected]) ABSTRACT The new combination Acmispon argophyllus (A.Gray) Brouillet var. niveus (Greene) Brouillet is made. Key words: Acmispon, California, Fabaceae, Loteae, North America, Santa Cruz Island. Acmispon argophyllus (A.Gray) Brouillet var. niveus (Greene) Variety niveus is a northern Channel Islands (California) Brouillet, comb. et stat. nov.—TYPE: California. Santa endemic that is distinguished from the closely related southern Cruz Island [s.d.], E.L. Greene s.n. (holotype CAS!, isotype Channel Islands endemic var. adsurgens (Dunkle) Brouillet by (part of type) UC!). stems ascending to erect (vs. erect), less crowded leaves, a silky (vs. silvery) indumentum, smaller umbels (6–10 vs. 10–13 Basionym: Syrmatium niveum Greene, Bull. Calif. Acad. Sci. 2: 148 flowers), and slightly longer calyx lobes (2.5–5.0 vs. -

Ventura County Planning Division 2018 Locally Important Plant List
Ventura County Planning Division 2018 Locally Important Plant List Number of Scientific Name Common Name Habit Family Federal/State Status Occurrences in Source Ventura County Abronia turbinata Torr. ex S. Consortium of California Turbinate Sand-verbena A/PH Nyctaginaceae 2 Watson Herbaria Acanthoscyphus parishii var. abramsii (E.A. McGregor) Consortium of California Abrams' Oxytheca AH Polygonaceae CRPR 1B.2 4-5 Reveal [synonym: Oxytheca Herbaria parishii var. abramsii] Acanthoscyphus parishii Consortium of California Parish Oxytheca AH Polygonaceae CRPR 4.2 1 (Parry) Small var. parishii Herbaria Acmispon glaber var. Consortium of California brevialatus (Ottley) Brouillet Short Deerweed PH Fabaceae 1 Herbaria Acmispon heermannii Heermann Lotus or Consortium of California (Durand & Hilg.) Brouillet var. PH Fabaceae 4 Hosackia Herbaria heermannii Acmispon heermannii var. Roundleaf Heermann Consortium of California PH Fabaceae 1 orbicularis (A. Gray) Brouillet Lotus or Hosackia Herbaria Acmispon junceus (Bentham) Consortium of California Rush Hosackia AH Fabaceae 2 Brouillet var. junceus Herbaria 1 Locally Important Plant List- Dec. 2018 Number of Scientific Name Common Name Habit Family Federal/State Status Occurrences in Source Ventura County Acmispon micranthus (Torrey Consortium of California Grab Hosackia or Lotus AH Fabaceae 3 & A. Gray) Brouillet Herbaria Acmispon parviflorus Consortium of California Tiny Lotus AH Fabaceae 2 (Bentham) D.D. Sokoloff Herbaria Consortium of California Agrostis hallii Vasey Hall's Bentgrass PG Poaceae 1 Herbaria Common or Broadleaf Consortium of California Alisma plantago-aquaticum L. PH Alismataceae 4 Water-plantain Herbaria Consortium of California Allium amplectens Torrey Narrowleaf Onion PG Alliaceae 1 Herbaria Allium denticulatum (Traub) Consortium of California Dentate Fringed Onion PG Alliaceae 1 D. -

Legally Listed Species of the California Central Coast Region (U S Fish and Wildlife Service and /Or the State of California)
Legally Listed Species of the California Central Coast Region (U S Fish and Wildlife Service and /or the State of California) (Monterey, San Benito, San Luis Obispo, western Kern, Santa Barbara, and Ventura counties) The following taxa, in alphabetical order by scientific name, are listed either by the U. S. Fish and Wildlife Service (Endangered Species Act) or by the State of California, Department of Fish and Wildlife, Natural Diversity Database. A comprehensive list for the State of California is updated quarterly by the California Natural Diversity Database. [Special Vascular Plants, Bryophytes, and Lichens List.] The distribution of these species has been documented for California’s central coast region from Monterey and San Benito counties south to Ventura County, and including western Kern County. Scientific names are those used in Baldwin et. al., 2012, The Jepson Manual: vascular plants of California, UC Press, Berkeley. Where nomenclature has changed from the name used initially in the listing process, they are referenced to the current name (e.g., Arabis hoffmannii = Boechera hoffmannii). Listing Status FE = federally endangered (Endangered Species Act, 1973 as amended) FT = federally threatened (Endangered Species Act, 1973 as amended) SE = state endangered (California Endangered Species Act; Fish and Game Code §2050 et seq.) ST = state threatened (California Endangered Species Act; Fish and Game Code §2050 et seq.) SR = state rare (California Native Plant Protection Act; Fish and Game Code §1900 et seq.). Scientific Name Common Name Status Acmispon argophyllus var. niveus Santa Cruz Island birds-foot trefoil SE Arabis hoffmannii = Boechera hoffmannii Boechera hoffmannii Hoffmann’s rock-cress FE Arctostaphylos confertiflora Santa Rosa Island manzanita FE Arctostaphylos hookeri subsp. -

Lotus Scoparius (Nutt. in Torr. & A. Gray) Ottley [= Acmispon Glaber
SPECIES Lotus scoparius (Nutt. in Torr. & A. Gray) Ottley [= Acmispon glaber (Vogel) Brouillet] NRCS CODE: Tribe: Loteae LOSC2 Subfamily: Papilionoideae LOSCS2 Family: Fabaceae LOSCB Order: Fabales Subclass: Rosidae Class: Magnoliopsida LOSCB , Riverside Co., A. Montalvo 2009 LOSCS2, Monterey coast, A. Montalvo 2003 LOSCB, Riverside Co., A. Montalvo 2010, Subspecific taxa 1. LOSCS2 1. Lotus scoparius var. scoparius 2. LOSCB 2. Lotus scoparius (Nutt.) Ottley var. brevialatus Ottley Synonyms 1. Acmispon glaber (Vogel) Brouillet var. glaber [New name in Jepson Manual 2nd Edition, JepsonOnline 2010] Hosackia scoparia Nutt. in T. and G. (taxa numbered as above) H. glaber Greene H. crassifolia Nutt., not Benth L. glaber Greene, not Mill. L. scoparius (Torr. & A. Gray) Ottley L. scoparius (Nutt. in T. & G.) Ottley ssp. scoparius (Ottley) Munz Lotus scoparius (Nutt.) Ottley var. perplexans Hoover p.p. Syrmatium glabrum Vogel 2. Acmispon glaber (Vogel) Brouillet var. brevialatus (Ottley) Brouillet [New name in Jepson Manual 2nd Edition] Hosackia glabra (Vogel) Torr. var. brevialata (Ottley) Abrams Lotus scoparius (Torr. & A. Gray) Ottley var. brevialatus Ottley Lotus scoparius (Nutt. in T. & G.) Ottley ssp. brevialatus (Ottley) Munz Common name General for species: California broom, deerweed 1. coastal deerweed, common deerweed (taxa numbered as above) 2. desert deerweed, western bird's foot trefoil, short-winged deerweed (Roberts 2008, Painter 2009, USDA PLANTS 2010). Over 45 taxa of Lotus were recognized in Isely's treatment in Hickman (1993) for California. These taxa had been grouped and regrouped into various species as well as subgenera or genera based on morphology for over a century. Allan & Porter (2000) analyzed DNA (ITS and nuclear ribosomal DNA), geographic, and morphological data for more than 45 taxa of Lotus together with additional related taxa of Loteae and found several geographically distinct lineages. -

Specialization-Generalization Trade-Off in a Bradyrhizobium Symbiosis with Wild Legume Hosts Ehinger Et Al
6 Test Host 5 A. strigosus L. bicolor 4 3 2 Growth response 1 0 A. strigosus L. bicolor Source species Specialization-generalization trade-off in a Bradyrhizobium symbiosis with wild legume hosts Ehinger et al. Ehinger et al. BMC Ecology 2014, 14:8 http://www.biomedcentral.com/1472-6785/14/8 Ehinger et al. BMC Ecology 2014, 14:8 http://www.biomedcentral.com/1472-6785/14/8 RESEARCH ARTICLE Open Access Specialization-generalization trade-off in a Bradyrhizobium symbiosis with wild legume hosts Martine Ehinger1, Toni J Mohr1, Juliana B Starcevich1, Joel L Sachs2,3, Stephanie S Porter1 and Ellen L Simms1* Abstract Background: Specialized interactions help structure communities, but persistence of specialized organisms is puzzling because a generalist can occupy more environments and partake in more beneficial interactions. The “Jack-of-all-trades is a master of none” hypothesis asserts that specialists persist because the fitness of a generalist utilizing a particular habitat is lower than that of a specialist adapted to that habitat. Yet, there are many reasons to expect that mutualists will generalize on partners. Plant-soil feedbacks help to structure plant and microbial communities, but how frequently are soil-based symbiotic mutualistic interactions sufficiently specialized to influence species distributions and community composition? To address this question, we quantified realized partner richness and phylogenetic breadth of four wild-grown native legumes (Lupinus bicolor, L. arboreus, Acmispon strigosus and A. heermannii) and performed inoculation trials to test the ability of two hosts (L. bicolor and A. strigosus) to nodulate (fundamental partner richness), benefit from (response specificity), and provide benefit to (effect specificity) 31 Bradyrhizobium genotypes. -
![Acmispon Glaber (Vogel) Brouillet [Updated 2017] = Lotus Scoparius (Nutt](https://docslib.b-cdn.net/cover/3383/acmispon-glaber-vogel-brouillet-updated-2017-lotus-scoparius-nutt-1833383.webp)
Acmispon Glaber (Vogel) Brouillet [Updated 2017] = Lotus Scoparius (Nutt
I. SPECIES Acmispon glaber (Vogel) Brouillet [Updated 2017] = Lotus scoparius (Nutt. in Torr. & A. Gray) Ottley NRCS CODE: [none for Tribe: Loteae Family: Fabaceae Subclass: Rosidae Acmispon] Subfamily: Papilionoideae Order: Fabales Class: Magnoliopsida [LOSC2 code for L. 4 mm scoparius ] Acmispon glaber var. brevialatus [= Lotus scoparius var. brevialatus] 15 mm seedling with linear cotyledons and first pair of true leaves 4 mm Acmispon glaber var. glaber erect form prostrate form on Monterey coast A. Subspecific taxa 1. no NRCS code 1. Acmispon glaber (Vogel) Brouillet var. glaber 2. no NRCS code 2. Acmispon glaber (Vogel) Brouillet var. brevialatus (Ottley) Brouillet [accepted by Baldwin et al. (2012), Jepson eFlora (2017)] B. Common name General for species: deerweed, California broom 1. coastal deerweed, common deerweed, deerweed, coastal deerbroom (taxa numbered as above; 2. short-winged deerweed, desert deerweed, desert deerbroom, western bird's foot trefoil names listed first used below) (Roberts 2008, Allen & Roberts 2013, Calflora 2016, USDA PLANTS 2016). Last modified: 10/19/2018 LOSC2 Update, 1 Printed: 10/19/2018 C. Synonyms 1. LOSCS2 1. Lotus scoparius (Nutt.) Ottley var. scoparius Hosackia scoparia Nutt. in T. and G. H. glaber Greene H. crassifolia Nutt., not Benth L. glaber Greene, not Mill. L. scoparius (Torr. & A. Gray) Ottley L. scoparius (Nutt. in T. & G.) Ottley ssp. scoparius (Ottley) Munz Lotus scoparius (Nutt.) Ottley var. perplexans Hoover p.p. Syrmatium glabrum Vogel 2. LOSCB 2. Lotus scoparius (Nutt.) Ottley var. brevialatus Ottley (taxa numbered as above) Hosackia glabra (Vogel) Torr. var. brevialata (Ottley) Abrams Lotus scoparius (Torr. & A. Gray) Ottley var. brevialatus Ottley Lotus scoparius (Nutt. -

Shared Flora of the Alta and Baja California Pacific Islands
Monographs of the Western North American Naturalist Volume 7 8th California Islands Symposium Article 12 9-25-2014 Island specialists: shared flora of the Alta and Baja California Pacific slI ands Sarah E. Ratay University of California, Los Angeles, [email protected] Sula E. Vanderplank Botanical Research Institute of Texas, 1700 University Dr., Fort Worth, TX, [email protected] Benjamin T. Wilder University of California, Riverside, CA, [email protected] Follow this and additional works at: https://scholarsarchive.byu.edu/mwnan Recommended Citation Ratay, Sarah E.; Vanderplank, Sula E.; and Wilder, Benjamin T. (2014) "Island specialists: shared flora of the Alta and Baja California Pacific slI ands," Monographs of the Western North American Naturalist: Vol. 7 , Article 12. Available at: https://scholarsarchive.byu.edu/mwnan/vol7/iss1/12 This Monograph is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Monographs of the Western North American Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Monographs of the Western North American Naturalist 7, © 2014, pp. 161–220 ISLAND SPECIALISTS: SHARED FLORA OF THE ALTA AND BAJA CALIFORNIA PACIFIC ISLANDS Sarah E. Ratay1, Sula E. Vanderplank2, and Benjamin T. Wilder3 ABSTRACT.—The floristic connection between the mediterranean region of Baja California and the Pacific islands of Alta and Baja California provides insight into the history and origin of the California Floristic Province. We present updated species lists for all California Floristic Province islands and demonstrate the disjunct distributions of 26 taxa between the Baja California and the California Channel Islands. -

UC Riverside UC Riverside Previously Published Works
UC Riverside UC Riverside Previously Published Works Title Fitness variation among host species and the paradox of ineffective rhizobia. Permalink https://escholarship.org/uc/item/0px6z7b1 Journal Journal of evolutionary biology, 31(4) ISSN 1010-061X Authors Pahua, VJ Stokes, PJN Hollowell, AC et al. Publication Date 2018-04-01 DOI 10.1111/jeb.13249 Peer reviewed eScholarship.org Powered by the California Digital Library University of California doi: 10.1111/jeb.13249 Fitness variation among host species and the paradox of ineffective rhizobia V. J. PAHUA*, P. J. N. STOKES†,A.C.HOLLOWELL*,J.U.REGUS*, K. A. GANO-COHEN*, C. E. WENDLANDT†,K.W.QUIDES*,J.Y.LYU*& J. L. SACHS*†‡ *Department of Evolution Ecology and Organismal Biology, University of California, Riverside, CA, USA †Department of Botany and Plant Sciences, University of California, Riverside, CA, USA ‡Institute for Integrative Genome Biology, University of California, Riverside, CA, USA Keywords: Abstract Acmispon strigosus; Legumes can preferentially select beneficial rhizobial symbionts and sanction Bradyrhizobium; ineffective strains that fail to fix nitrogen. Yet paradoxically, rhizobial popu- cheater symbionts; lations vary from highly beneficial to ineffective in natural and agricultural host control; soils. Classic models of symbiosis focus on the single dimension of symbiont ineffective symbionts; cost-benefit to sympatric hosts, but fail to explain the widespread persistence trade-offs. of ineffective rhizobia. Here, we test a novel framework predicting that spa- tio-temporal and community dynamics can maintain ineffective strains in rhizobial populations. We used clonal and multistrain inoculations and quantitative culturing to investigate the relative fitness of four focal Bradyrhizobium strains varying from effective to ineffective on Acmispon strigosus. -
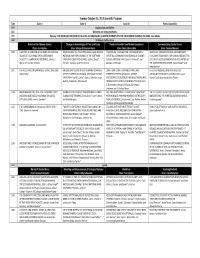
Tuesday October 23, 2012 Scientific Program
Tuesday October 23, 2012 Scientific Program Time Salon I Salon II Salon III Puerto Escondido 0800 Registration and Coffee 0845 Welcome and Announcements 0900 Plenary: THE GEOLOGIC RECORD OF GLACIAL‐INTERGLACIAL CLIMATE CHANGES ON THE CALIFORNIA CHANNEL ISLANDS‐ Dan Muhs 1000 30 Minute Coffee Break History of the Channel Islands Changes in Assemblages of Flora and Fauna Trends in Intertidal and Marine Ecosystems Communicating Coastal Issues Chair: Ann Huston Chair: Stacey Ostermann‐Kelm Chair: Mary Elaine Helix Chair: Yvonne Menard 1030 A HISTORY OF AVIATION IN THE NORTHERN CHANNEL DOCUMENTING THE PALEONTOLOGICAL RESOURCES OF TIDEPOOLING THROUGH TIME: 30 YEARS OF ROCKY SANCTUARY ADVISORY COUNCIL AND SANCTUARY ISLANDS OF CALIFORNIA: FROM GENTLEMEN'S NATIONAL PARK SERVICE AREAS OF THE SOUTHERN INTERTIDAL COMMUNITY MONITORING AT CHANNEL EDUCATION TEAM MODEL FOR COMMUNICATING THE NOVELTY TO COMMERCIAL ENTERPRISE. Charles J CALIFORNIA COAST AND ISLANDS. Justin S. Tweet*, ISLANDS NATIONAL PARK. Daniel V. Richards* and EFFECTS OF OCEAN ACIDIFICATION IN THE WATERS OF Rennie, III* and Ian Williams Vincent L. Santucci, and Tim Connors Stephen G. Whitaker THE SANTA BARBARA CHANNEL. Maria Petueli* and Amanda Allen 1045 THE MILLS YEARS ON SAN MIGUEL ISLAND, 1869‐1888. AN 8000 YEAR RECORD OF BIRD REMAINS FROM CAVE LONG‐TERM CLIMATE VARIABILITY AND LINKS EFFECTIVE MESSAGES AND STRATIGIES FOR Marla Daily* OF THE CHIMNEYS (CA‐SMI‐603), SAN MIGUEL ISLAND, BETWEEN PATTERNS OF COASTAL MARINE COMMUNICATING OCEAN ACIDIFICATION. Laura CALIFORNIA. Paul W. Collins*, Emily L. Whistler, Daniel INVERTEBRATE RECRUITMENT AND ADULT ABUNDANCE Francis*, Julie Bursek and Claire Fackler Guthrie, and René L. Vellanoweth AROUND SANTA CRUZ ISLAND FROM 1995‐2010. -

(POWO) Rafaël H.A. Govaerts
Skvortsovia: 4(3): 74 –99 (2018) Skvortsovia ISSN 2309-6497 (Print) Copyright: © 2018 Russian Academy of Sciences http://skvortsovia.uran.ru/ ISSN 2309-6500 (Online) Article 101 Nomenclatural Corrections in Preparation for the Plants of the World Online (POWO) Rafaël H.A. Govaerts Royal Botanic Gardens, Kew. Richmond. Surrey. TW9 3AE, United Kingdom. E-mail: [email protected] Received: 23 July 2018 | Accepted by Wendy Applequist: 05 November 2018 | Published on line: 29 November 2018 Abstract In preparation for Plants of the World Online (POWO), 101 new combinations and replacement names are published and also names in current use are validated, including 30 infraspecific names in the genus Phylica L. Keywords: new combinations, nomenclature, POWO, replacement names, valid publication Introduction The 2020 target 1 of the Convention for Botanical Diversity (CBD) is to create “An online flora of all known plants.” Kew’s answer to this is the Plants of the World Online (hereafter, POWO, http://plantsoftheworldonline.org/). POWO draws together Kew’s extensive data resources including its regional floras and monographs, alongside images from the digitisation of the collections. These data will then feed into the World Flora Online (WFO, http://www.worldfloraonline.org/) consortium. The taxonomic backbone of POWO is the World Checklist of Vascular Plants (WCVP) dataset, which is partly available online through the World Checklist of Selected Plant Families (WCSP) website (http://wcsp.science.kew.org/). The data compilation of this programme was started in 1988 and many nomenclatural issues were dealt with, sometimes resulting in new combinations and replacement names being indicated as “ined.” (ineditus [Latin] – unpublished) in the database and now visible online. -

Checklist of the Vascular Plants of San Diego County 5Th Edition
cHeckliSt of tHe vaScUlaR PlaNtS of SaN DieGo coUNty 5th edition Pinus torreyana subsp. torreyana Downingia concolor var. brevior Thermopsis californica var. semota Pogogyne abramsii Hulsea californica Cylindropuntia fosbergii Dudleya brevifolia Chorizanthe orcuttiana Astragalus deanei by Jon P. Rebman and Michael G. Simpson San Diego Natural History Museum and San Diego State University examples of checklist taxa: SPecieS SPecieS iNfRaSPecieS iNfRaSPecieS NaMe aUtHoR RaNk & NaMe aUtHoR Eriodictyon trichocalyx A. Heller var. lanatum (Brand) Jepson {SD 135251} [E. t. subsp. l. (Brand) Munz] Hairy yerba Santa SyNoNyM SyMBol foR NoN-NATIVE, NATURaliZeD PlaNt *Erodium cicutarium (L.) Aiton {SD 122398} red-Stem Filaree/StorkSbill HeRBaRiUM SPeciMeN coMMoN DocUMeNTATION NaMe SyMBol foR PlaNt Not liSteD iN THE JEPSON MANUAL †Rhus aromatica Aiton var. simplicifolia (Greene) Conquist {SD 118139} Single-leaF SkunkbruSH SyMBol foR StRict eNDeMic TO SaN DieGo coUNty §§Dudleya brevifolia (Moran) Moran {SD 130030} SHort-leaF dudleya [D. blochmaniae (Eastw.) Moran subsp. brevifolia Moran] 1B.1 S1.1 G2t1 ce SyMBol foR NeaR eNDeMic TO SaN DieGo coUNty §Nolina interrata Gentry {SD 79876} deHeSa nolina 1B.1 S2 G2 ce eNviRoNMeNTAL liStiNG SyMBol foR MiSiDeNtifieD PlaNt, Not occURRiNG iN coUNty (Note: this symbol used in appendix 1 only.) ?Cirsium brevistylum Cronq. indian tHiStle i checklist of the vascular plants of san Diego county 5th edition by Jon p. rebman and Michael g. simpson san Diego natural history Museum and san Diego state university publication of: san Diego natural history Museum san Diego, california ii Copyright © 2014 by Jon P. Rebman and Michael G. Simpson Fifth edition 2014. isBn 0-918969-08-5 Copyright © 2006 by Jon P.