Structure, Dielectric and Multiferroic Properties of Bi0.85Nd0.15Fe0.98Zr0.02O3 in Ba and Ti Co- Doping
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

De Sousa Sinitic MSEA
THE FAR SOUTHERN SINITIC LANGUAGES AS PART OF MAINLAND SOUTHEAST ASIA (DRAFT: for MPI MSEA workshop. 21st November 2012 version.) Hilário de Sousa ERC project SINOTYPE — École des hautes études en sciences sociales [email protected]; [email protected] Within the Mainland Southeast Asian (MSEA) linguistic area (e.g. Matisoff 2003; Bisang 2006; Enfield 2005, 2011), some languages are said to be in the core of the language area, while others are said to be periphery. In the core are Mon-Khmer languages like Vietnamese and Khmer, and Kra-Dai languages like Lao and Thai. The core languages generally have: – Lexical tonal and/or phonational contrasts (except that most Khmer dialects lost their phonational contrasts; languages which are primarily tonal often have five or more tonemes); – Analytic morphological profile with many sesquisyllabic or monosyllabic words; – Strong left-headedness, including prepositions and SVO word order. The Sino-Tibetan languages, like Burmese and Mandarin, are said to be periphery to the MSEA linguistic area. The periphery languages have fewer traits that are typical to MSEA. For instance, Burmese is SOV and right-headed in general, but it has some left-headed traits like post-nominal adjectives (‘stative verbs’) and numerals. Mandarin is SVO and has prepositions, but it is otherwise strongly right-headed. These two languages also have fewer lexical tones. This paper aims at discussing some of the phonological and word order typological traits amongst the Sinitic languages, and comparing them with the MSEA typological canon. While none of the Sinitic languages could be considered to be in the core of the MSEA language area, the Far Southern Sinitic languages, namely Yuè, Pínghuà, the Sinitic dialects of Hǎinán and Léizhōu, and perhaps also Hakka in Guǎngdōng (largely corresponding to Chappell (2012, in press)’s ‘Southern Zone’) are less ‘fringe’ than the other Sinitic languages from the point of view of the MSEA linguistic area. -

Safety of Chinese Herbal Medicine
SAFETY OF CHINESE HERBAL MEDICINE Giovanni Maciocia® Su Wen Press 2003 GIOVANNI MACIOCIA Published in 1999 by Su Wen Press 5 Buckingham House Bois Lane Chesham Bois Buckinghamshire UK Copyright © Giovanni Maciocia All rights reserved, including translation. No part of this publication may be reproduced or transmitted by any means, electronic or mechanical, recording or duplication in any information or storage and retrieval system without permission in writing from the publishers, and may not be photocopied or otherwise reproduced even within the terms of any licence issued by the Copyright Licensing Agency Ltd. ISBN 0 9536157 0 7 First edition 1999 Second edition 2000 Third edition 2003 2 GIOVANNI MACIOCIA Contents Page Introduction 4 1. How drugs are metabolized and excreted ........................................................ 5 2. Factors affecting dosage of drugs ........................................................................ 10 3. Description of side-effects, adverse reactions, idiosyncratic ........... 12 reactions and allergic reactions to drugs 4. Differences in the pharmacodynamics of drugs and herbs ................ 14 5. Side-effects, adverse reactions, idiosyncratic reactions ...................... 16 and allergic reactions to herbal medicines: a review of the literature with identification of some mistakes 6. Interactions between drugs and Chinese herbs ......................................... 21 7. Side-effects of Chinese herbal formulae and how .................................... 27 to deal with them 8. Symptoms -

中国人的姓名 王海敏 Wang Hai Min
中国人的姓名 王海敏 Wang Hai min last name first name Haimin Wang 王海敏 Chinese People’s Names Two parts Last name First name 姚明 Yao Ming Last First name name Jackie Chan 成龙 cheng long Last First name name Bruce Lee 李小龙 li xiao long Last First name name The surname has roughly several origins as follows: 1. the creatures worshipped in remote antiquity . 龙long, 马ma, 牛niu, 羊yang, 2. ancient states’ names 赵zhao, 宋song, 秦qin, 吴wu, 周zhou 韩han,郑zheng, 陈chen 3. an ancient official titles 司马sima, 司徒situ 4. the profession. 陶tao,钱qian, 张zhang 5. the location and scene in residential places 江jiang,柳 liu 6.the rank or title of nobility 王wang,李li • Most are one-character surnames, but some are compound surname made up of two of more characters. • 3500Chinese surnames • 100 commonly used surnames • The three most common are 张zhang, 王wang and 李li What does my name mean? first name strong beautiful lively courageous pure gentle intelligent 1.A person has an infant name and an official one. 2.In the past,the given names were arranged in the order of the seniority in the family hierarchy. 3.It’s the Chinese people’s wish to give their children a name which sounds good and meaningful. Project:Search on-Line www.Mandarinintools.com/chinesename.html Find Chinese Names for yourself, your brother, sisters, mom and dad, or even your grandparents. Find meanings of these names. ----What is your name? 你叫什么名字? ni jiao shen me ming zi? ------ 我叫王海敏 wo jiao Wang Hai min ------ What is your last name? 你姓什么? ni xing shen me? (你贵姓?)ni gui xing? ------ 我姓 王,王海敏。 wo xing wang, Wang Hai min ----- What is your nationality? 你是哪国人? ni shi na guo ren? ----- I am chinese/American 我是中国人/美国人 Wo shi zhong guo ren/mei guo ren 百家 姓 bai jia xing 赵(zhào) 钱(qián) 孙(sūn) 李(lǐ) 周(zhōu) 吴(wú) 郑(zhèng) 王(wán 冯(féng) 陈(chén) 褚(chǔ) 卫(wèi) 蒋(jiǎng) 沈(shěn) 韩(hán) 杨(yáng) 朱(zhū) 秦(qín) 尤(yóu) 许(xǔ) 何(hé) 吕(lǚ) 施(shī) 张(zhāng). -

The Case of Wang Wei (Ca
_full_journalsubtitle: International Journal of Chinese Studies/Revue Internationale de Sinologie _full_abbrevjournaltitle: TPAO _full_ppubnumber: ISSN 0082-5433 (print version) _full_epubnumber: ISSN 1568-5322 (online version) _full_issue: 5-6_full_issuetitle: 0 _full_alt_author_running_head (neem stramien J2 voor dit article en vul alleen 0 in hierna): Sufeng Xu _full_alt_articletitle_deel (kopregel rechts, hier invullen): The Courtesan as Famous Scholar _full_is_advance_article: 0 _full_article_language: en indien anders: engelse articletitle: 0 _full_alt_articletitle_toc: 0 T’OUNG PAO The Courtesan as Famous Scholar T’oung Pao 105 (2019) 587-630 www.brill.com/tpao 587 The Courtesan as Famous Scholar: The Case of Wang Wei (ca. 1598-ca. 1647) Sufeng Xu University of Ottawa Recent scholarship has paid special attention to late Ming courtesans as a social and cultural phenomenon. Scholars have rediscovered the many roles that courtesans played and recognized their significance in the creation of a unique cultural atmosphere in the late Ming literati world.1 However, there has been a tendency to situate the flourishing of late Ming courtesan culture within the mainstream Confucian tradition, assuming that “the late Ming courtesan” continued to be “integral to the operation of the civil-service examination, the process that re- produced the empire’s political and cultural elites,” as was the case in earlier dynasties, such as the Tang.2 This assumption has suggested a division between the world of the Chinese courtesan whose primary clientele continued to be constituted by scholar-officials until the eight- eenth century and that of her Japanese counterpart whose rise in the mid- seventeenth century was due to the decline of elitist samurai- 1) For important studies on late Ming high courtesan culture, see Kang-i Sun Chang, The Late Ming Poet Ch’en Tzu-lung: Crises of Love and Loyalism (New Haven: Yale Univ. -

Ideophones in Middle Chinese
KU LEUVEN FACULTY OF ARTS BLIJDE INKOMSTSTRAAT 21 BOX 3301 3000 LEUVEN, BELGIË ! Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas'Van'Hoey' ' Presented(in(fulfilment(of(the(requirements(for(the(degree(of(( Master(of(Arts(in(Linguistics( ( Supervisor:(prof.(dr.(Jean=Christophe(Verstraete((promotor)( ( ( Academic(year(2014=2015 149(431(characters Abstract (English) Ideophones in Middle Chinese: A Typological Study of a Tang Dynasty Poetic Corpus Thomas Van Hoey This M.A. thesis investigates ideophones in Tang dynasty (618-907 AD) Middle Chinese (Sinitic, Sino- Tibetan) from a typological perspective. Ideophones are defined as a set of words that are phonologically and morphologically marked and depict some form of sensory image (Dingemanse 2011b). Middle Chinese has a large body of ideophones, whose domains range from the depiction of sound, movement, visual and other external senses to the depiction of internal senses (cf. Dingemanse 2012a). There is some work on modern variants of Sinitic languages (cf. Mok 2001; Bodomo 2006; de Sousa 2008; de Sousa 2011; Meng 2012; Wu 2014), but so far, there is no encompassing study of ideophones of a stage in the historical development of Sinitic languages. The purpose of this study is to develop a descriptive model for ideophones in Middle Chinese, which is compatible with what we know about them cross-linguistically. The main research question of this study is “what are the phonological, morphological, semantic and syntactic features of ideophones in Middle Chinese?” This question is studied in terms of three parameters, viz. the parameters of form, of meaning and of use. -

Mr. Bai and Mr. Qian Earntheir Living: Considering Two Handwritten Notebooks of Matching Couplets from China in the Late Qing An
chapter 7 Mr. Bai and Mr. Qian Earn Their Living: Considering Two Handwritten Notebooks of Matching Couplets from China in the Late Qing and Early Republic 老白老錢寫對聯兒 Introduction This chapter explores two chaoben that I bought in China in the spring of 2012. They were written between the end of the Qing dynasty, around 1880, and the early years of the Republic, about 1913. They were both written by literate men whose daily life was very close to the common people in terms of their life expectations and their modest incomes. The men, who had received some formal education and had the ability to read and to write, took advantage of those abilities to earn income by preparing couplets of congratulatory poems that were, and still are, a big part of the social and celebratory life of the Chinese. By attempting to draw inferences and make logical assumptions from the information in these two chaoben and the manner in which the information was presented, I suggest the ways in which they can reveal something about the lives, the worldview, and the economic standing of both men. By looking carefully at the contents of what they wrote, we see that these chaoben can also be used to reconstruct something about the communities in which both men lived and worked. The bulk of the content in both chaoben is matching couplets. Many literate men in this period could regularly earn extra income by offering to write matching couplets, celebratory rhyming phrases that were demanded on many social occasions, such as New Year festivities and weddings. -

Daily Life for the Common People of China, 1850 to 1950
Daily Life for the Common People of China, 1850 to 1950 Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access China Studies published for the institute for chinese studies, university of oxford Edited by Micah Muscolino (University of Oxford) volume 39 The titles published in this series are listed at brill.com/chs Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access Daily Life for the Common People of China, 1850 to 1950 Understanding Chaoben Culture By Ronald Suleski leiden | boston Ronald Suleski - 978-90-04-36103-4 Downloaded from Brill.com04/05/2019 09:12:12AM via free access This is an open access title distributed under the terms of the prevailing cc-by-nc License at the time of publication, which permits any non-commercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. An electronic version of this book is freely available, thanks to the support of libraries working with Knowledge Unlatched. More information about the initiative can be found at www.knowledgeunlatched.org. Cover Image: Chaoben Covers. Photo by author. Library of Congress Cataloging-in-Publication Data Names: Suleski, Ronald Stanley, author. Title: Daily life for the common people of China, 1850 to 1950 : understanding Chaoben culture / By Ronald Suleski. -

Origin Narratives: Reading and Reverence in Late-Ming China
Origin Narratives: Reading and Reverence in Late-Ming China Noga Ganany Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2018 © 2018 Noga Ganany All rights reserved ABSTRACT Origin Narratives: Reading and Reverence in Late Ming China Noga Ganany In this dissertation, I examine a genre of commercially-published, illustrated hagiographical books. Recounting the life stories of some of China’s most beloved cultural icons, from Confucius to Guanyin, I term these hagiographical books “origin narratives” (chushen zhuan 出身傳). Weaving a plethora of legends and ritual traditions into the new “vernacular” xiaoshuo format, origin narratives offered comprehensive portrayals of gods, sages, and immortals in narrative form, and were marketed to a general, lay readership. Their narratives were often accompanied by additional materials (or “paratexts”), such as worship manuals, advertisements for temples, and messages from the gods themselves, that reveal the intimate connection of these books to contemporaneous cultic reverence of their protagonists. The content and composition of origin narratives reflect the extensive range of possibilities of late-Ming xiaoshuo narrative writing, challenging our understanding of reading. I argue that origin narratives functioned as entertaining and informative encyclopedic sourcebooks that consolidated all knowledge about their protagonists, from their hagiographies to their ritual traditions. Origin narratives also alert us to the hagiographical substrate in late-imperial literature and religious practice, wherein widely-revered figures played multiple roles in the culture. The reverence of these cultural icons was constructed through the relationship between what I call the Three Ps: their personas (and life stories), the practices surrounding their lore, and the places associated with them (or “sacred geographies”). -

Representing Talented Women in Eighteenth-Century Chinese Painting: Thirteen Female Disciples Seeking Instruction at the Lake Pavilion
REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION By Copyright 2016 Janet C. Chen Submitted to the graduate degree program in Art History and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson Marsha Haufler ________________________________ Amy McNair ________________________________ Sherry Fowler ________________________________ Jungsil Jenny Lee ________________________________ Keith McMahon Date Defended: May 13, 2016 The Dissertation Committee for Janet C. Chen certifies that this is the approved version of the following dissertation: REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION ________________________________ Chairperson Marsha Haufler Date approved: May 13, 2016 ii Abstract As the first comprehensive art-historical study of the Qing poet Yuan Mei (1716–97) and the female intellectuals in his circle, this dissertation examines the depictions of these women in an eighteenth-century handscroll, Thirteen Female Disciples Seeking Instructions at the Lake Pavilion, related paintings, and the accompanying inscriptions. Created when an increasing number of women turned to the scholarly arts, in particular painting and poetry, these paintings documented the more receptive attitude of literati toward talented women and their support in the social and artistic lives of female intellectuals. These pictures show the women cultivating themselves through literati activities and poetic meditation in nature or gardens, common tropes in portraits of male scholars. The predominantly male patrons, painters, and colophon authors all took part in the formation of the women’s public identities as poets and artists; the first two determined the visual representations, and the third, through writings, confirmed and elaborated on the designated identities. -

How Do Water Molecules Form a Hydrogen Bonding Network? Weiduo Zhu University of Science and Technology of China
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 2016 Two-dimensional interlocked pentagonal bilayer ice: how do water molecules form a hydrogen bonding network? Weiduo Zhu University of Science and Technology of China Wen- Hui Zhao University of Science and Technology of China Lu Wang University of Science and Technology of China Di Yin University of Science and Technology of China Min Jia University of Science and Technology of China See next page for additional authors Follow this and additional works at: http://digitalcommons.unl.edu/chemzeng Part of the Analytical Chemistry Commons, Materials Chemistry Commons, and the Physical Chemistry Commons Zhu, Weiduo; Zhao, Wen- Hui; Wang, Lu; Yin, Di; Jia, Min; Yang, Jinlong; Zeng, Xiao Cheng; and Yuan, Lan-Feng, "Two-dimensional interlocked pentagonal bilayer ice: how do water molecules form a hydrogen bonding network?" (2016). Xiao Cheng Zeng Publications. 148. http://digitalcommons.unl.edu/chemzeng/148 This Article is brought to you for free and open access by the Published Research - Department of Chemistry at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Xiao Cheng Zeng Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Weiduo Zhu, Wen- Hui Zhao, Lu Wang, Di Yin, Min Jia, Jinlong Yang, Xiao Cheng Zeng, and Lan-Feng Yuan This article is available at DigitalCommons@University of Nebraska - Lincoln: http://digitalcommons.unl.edu/chemzeng/148 Showcasing research from the laboratory of Dr Lan-Feng Yuan As featured in: and Prof. Xiao Cheng Zeng at University of Science and Technology of China Title: Two-dimensional interlocked pentagonal bilayer ice: how do water molecules form a hydrogen bonding network? An ideal H-bonding network of water should satisfy three requirements: four H-bonds per molecule, linear H-bonds, and tetrahedral configuration around each O atom. -
CHP-225 Terms
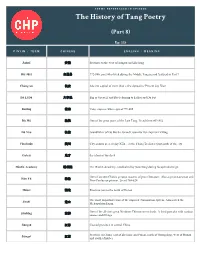
The History of Tang Poetry (Part 8) Ep. 225 Ānhuī 安徽 Province to the west of Jiangsu and Zhejiang Bái Jūyì 白居易 772-846, poet who lived during the Middle Tang period featured in Part 7 Cháng’an 长安 Ancient capital of more than a few dynasties/ Present day Xian Dà Lǐ Dù 大李杜 Big or Great Lǐ and Dù (referring to Li Bai and Du Fu) Dézōng 德宗 Tang emperor who reigned 779-805 Dù Mù 杜牧 One of the great poets of the Late Tang. Lived from 803-852 Dù Yòu 杜佑 Grandfather of Du Mu the Grand Councilor for emperor Dézōng Fánchuān 樊川 City around present day Xīān…in the Cháng’ān district just south of the city Guǐcái 鬼才 the talent of the devil Hànlín Academy 翰林院 The Hanlin Academy, established by Xuanzong during his splendid reign One of ancient China's greatest masters of prose literature. Also a great statesman and Hán Yù 韩愈 Neo-Confucian pioneer. Lived 768-824 Húběi 湖北 Province just to the north of Hunan The most important exam of the imperial examination system. Also called the Jinshi 进士 Metropolitan Exam One of the all-time great Northern Chinese street foods. A fried pancake with various jiānbǐng 煎餅 sauces and fillings Jiāngsū 江苏 Coastal province in central China Province in China, east of Zhejiang and Fujian, north of Guangdong, west of Hunan Jiāngxī 江西 and south of Hubei A poem of four lines of five to seven characters per line, with a strict tonal pattern and juéjù 绝句 rhyme scheme. Also known as a quatrain The second era of Xuanzong's reign, 713-741. -

Zeng Fanzhi Bibliography
G A G O S I A N Zeng Fanzhi Bibliography Selected Books and Catalogues: 2021 Chung, Gladys ed. Zeng Fanzhi Catalogue Raisonné: Volume I. Milan: Skira. 2016 Zeng, Fanzhi and Yuko Hasegawa. Zeng Fanzhi. New York: Gagosian Gallery. 2012 Zeng, Fanzhi, Zai-Fu and Robert E. Harris. Zeng Fanzhi. London: Gagosian Gallery. 2011 Zeng, Fanzhi. Zeng Fanzhi. Hong Kong: Gagosian Gallery. 2010 Zeng, Fanzhi, Fabien Fryns, and Richard Shift. Every Mark its Mask. Ostfildern: Hatje Cantz. 2009 Zeng, Fanzhi and William R. Acquavella. Zeng Fanzhi: Acquavella Contemporary Art. New York: Acquavella Contemporary Art. Zeng, Fanzhi. Zeng Fanzhi: April 2 – May 16, 2009: Acquavella Contemporary Art. New York: Acquavella Contemporary Art. 2007 Zeng, Fanzhi. Zeng Fanzhi, 1989-2007. Seoul, Korea: Gallery Hyundai. Zeng, Fanzhi; Lóránd Hegyi, and Peng Lu. Zeng Fanzhi. Saint-Etienne: Musée d'Art Moderne, Paris. 2006 Zeng, Fanzhi. The Paintings of Zeng Fanzhi. Shanghai: ShanghART. Selected Articles and Reviews: 2017 Zhao, William and Madeline Ross. “Masters of Reinvention.” Hong Kong Tatler, February 28. 2016 Qi, Lin. “Zeng show a time-travel retrospective.” China Daily, Aug 4. Qin, Amy. “Artist Zeng Fanzhi on the Evolution of His Work and China’s Art Market.” The New York Times, Sep 22. ---. “As China rises, top-selling painter looks to his roots.” France 24. October, 6. Tsui, Enid. “How China’s most bankable artist, Zeng Fanzhi, found new way to make art.” South China Morning Post. October 7. Chung, Stephy. “The man behind China’s million-dollar masks cares about art, not auctions.” CNN, October 20. Perlez, Jane. “How Zeng Fanzhi Became China’s Hottest Artist, and Why His Lawn Is So Green.” The New York Times, November 11.