Threat Diversity and Intensification on Islands Push Large Island Fruit Bats to the Brink
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bat Conservation 2021
Bat Conservation Global evidence for the effects of interventions 2021 Edition Anna Berthinussen, Olivia C. Richardson & John D. Altringham Conservation Evidence Series Synopses 2 © 2021 William J. Sutherland This document should be cited as: Berthinussen, A., Richardson O.C. and Altringham J.D. (2021) Bat Conservation: Global Evidence for the Effects of Interventions. Conservation Evidence Series Synopses. University of Cambridge, Cambridge, UK. Cover image: Leucistic lesser horseshoe bat Rhinolophus hipposideros hibernating in a former water mill, Wales, UK. Credit: Thomas Kitching Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ 3 Contents Advisory Board.................................................................................... 11 About the authors ............................................................................... 12 Acknowledgements ............................................................................. 13 1. About this book ........................................................... 14 1.1 The Conservation Evidence project ................................................................................. 14 1.2 The purpose of Conservation Evidence synopses ............................................................ 14 1.3 Who this synopsis is for ................................................................................................... 15 1.4 Background ..................................................................................................................... -

Download Book of Abstracts IB2019
BOOK OF ABSTRACTS Université de la Réunion Campus du Moufia 2 Island Biology Third International Conference on Island Ecology, Evolution and Conservation 8-13 July 2019 University of La R´eunion Saint Denis, France Book of Abstracts Editors: Olivier Flores Claudine Ah-Peng Nicholas Wilding Description of contents This document contains the collection of abstracts describing the research works presented at the third international conference on island ecology, evolution and conservation, Island Biology 2019, held in Saint Denis (La R´eunion,8-13 July 2019). In the following order, the different parts of this document concern Plenary sessions, Symposia, Regular sessions, and Poster presentations organized in thematic sections. The last part of the document consists of an author index with the names of all authors and links to the corresponding abstracts. Each abstract is referenced by a unique number 6-digits number indicated at the bottom of each page on the right. This reference number points to the online version of the abstract on the conference website using URL https://sciencesconf.org:ib2019/xxxxxx, where xxxxxx is the reference of the abstract. 4 Table of contents Plenary Sessions 19 Introduction to natural history of the Mascarene islands, Dominique Strasberg . 20 The history, current status, and future of the protected areas of Madagascar, Steven M. Goodman............................................ 21 The island biogeography of alien species, Tim Blackburn.................. 22 What can we learn about invasion ecology from ant invasions of islands ?, Lori Lach . 23 Orchids, moths, and birds on Madagascar, Mauritius, and Reunion: island systems with well-constrained timeframes for species interactions and trait change, Susanne Renner . -

Ctz78-02 (02) Lee Et Al.Indd 51 14 08 2009 13:12 52 Lee Et Al
Contributions to Zoology, 78 (2) 51-64 (2009) Variation in the nocturnal foraging distribution of and resource use by endangered Ryukyu flying foxes(Pteropus dasymallus) on Iriomotejima Island, Japan Ya-Fu Lee1, 4, Tokushiro Takaso2, 5, Tzen-Yuh Chiang1, 6, Yen-Min Kuo1, 7, Nozomi Nakanishi2, 8, Hsy-Yu Tzeng3, 9, Keiko Yasuda2 1 Department of Life Sciences and Institute of Biodiversity, National Cheng Kung University, Tainan 701, Taiwan 2 The Iriomote Project, Research Institute for Humanity and Nature, 671 Iriomote, Takatomi-cho, Okinawa 907- 1542, Japan 3 Hengchun Research Center, Taiwan Forestry Research Institute, Pingtung 946, Taiwan 4 E-mail: [email protected] 5 E-mail: [email protected] 6 E-mail: [email protected] 7 E-mail: [email protected] 8 E-mail: [email protected] 9 E-mail: [email protected] Key words: abundance, bats, Chiroptera, diet, figs, frugivores, habitat Abstract Contents The nocturnal distribution and resource use by Ryukyu flying foxes Introduction ........................................................................................ 51 was studied along 28 transects, covering five types of habitats, on Material and methods ........................................................................ 53 Iriomote Island, Japan, from early June to late September, 2005. Study sites ..................................................................................... 53 Bats were mostly encountered solitarily (66.8%) or in pairs (16.8%), Bat and habitat census ................................................................ -

Checklist of the Mammals of Indonesia
CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation i ii CHECKLIST OF THE MAMMALS OF INDONESIA Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation By Ibnu Maryanto Maharadatunkamsi Anang Setiawan Achmadi Sigit Wiantoro Eko Sulistyadi Masaaki Yoneda Agustinus Suyanto Jito Sugardjito RESEARCH CENTER FOR BIOLOGY INDONESIAN INSTITUTE OF SCIENCES (LIPI) iii © 2019 RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI) Cataloging in Publication Data. CHECKLIST OF THE MAMMALS OF INDONESIA: Scientific, English, Indonesia Name and Distribution Area Table in Indonesia Including CITES, IUCN and Indonesian Category for Conservation/ Ibnu Maryanto, Maharadatunkamsi, Anang Setiawan Achmadi, Sigit Wiantoro, Eko Sulistyadi, Masaaki Yoneda, Agustinus Suyanto, & Jito Sugardjito. ix+ 66 pp; 21 x 29,7 cm ISBN: 978-979-579-108-9 1. Checklist of mammals 2. Indonesia Cover Desain : Eko Harsono Photo : I. Maryanto Third Edition : December 2019 Published by: RESEARCH CENTER FOR BIOLOGY, INDONESIAN INSTITUTE OF SCIENCES (LIPI). Jl Raya Jakarta-Bogor, Km 46, Cibinong, Bogor, Jawa Barat 16911 Telp: 021-87907604/87907636; Fax: 021-87907612 Email: [email protected] . iv PREFACE TO THIRD EDITION This book is a third edition of checklist of the Mammals of Indonesia. The new edition provides remarkable information in several ways compare to the first and second editions, the remarks column contain the abbreviation of the specific island distributions, synonym and specific location. Thus, in this edition we are also corrected the distribution of some species including some new additional species in accordance with the discovery of new species in Indonesia. -

Island Biology Island Biology
IIssllaanndd bbiioollooggyy Allan Sørensen Allan Timmermann, Ana Maria Martín González Camilla Hansen Camille Kruch Dorte Jensen Eva Grøndahl, Franziska Petra Popko, Grete Fogtmann Jensen, Gudny Asgeirsdottir, Hubertus Heinicke, Jan Nikkelborg, Janne Thirstrup, Karin T. Clausen, Karina Mikkelsen, Katrine Meisner, Kent Olsen, Kristina Boros, Linn Kathrin Øverland, Lucía de la Guardia, Marie S. Hoelgaard, Melissa Wetter Mikkel Sørensen, Morten Ravn Knudsen, Pedro Finamore, Petr Klimes, Rasmus Højer Jensen, Tenna Boye Tine Biedenweg AARHUS UNIVERSITY 2005/ESSAYS IN EVOLUTIONARY ECOLOGY Teachers: Bodil K. Ehlers, Tanja Ingversen, Dave Parker, MIchael Warrer Larsen, Yoko L. Dupont & Jens M. Olesen 1 C o n t e n t s Atlantic Ocean Islands Faroe Islands Kent Olsen 4 Shetland Islands Janne Thirstrup 10 Svalbard Linn Kathrin Øverland 14 Greenland Eva Grøndahl 18 Azores Tenna Boye 22 St. Helena Pedro Finamore 25 Falkland Islands Kristina Boros 29 Cape Verde Islands Allan Sørensen 32 Tristan da Cunha Rasmus Højer Jensen 36 Mediterranean Islands Corsica Camille Kruch 39 Cyprus Tine Biedenweg 42 Indian Ocean Islands Socotra Mikkel Sørensen 47 Zanzibar Karina Mikkelsen 50 Maldives Allan Timmermann 54 Krakatau Camilla Hansen 57 Bali and Lombok Grete Fogtmann Jensen 61 Pacific Islands New Guinea Lucía de la Guardia 66 2 Solomon Islands Karin T. Clausen 70 New Caledonia Franziska Petra Popko 74 Samoa Morten Ravn Knudsen 77 Tasmania Jan Nikkelborg 81 Fiji Melissa Wetter 84 New Zealand Marie S. Hoelgaard 87 Pitcairn Katrine Meisner 91 Juan Fernandéz Islands Gudny Asgeirsdottir 95 Hawaiian Islands Petr Klimes 97 Galápagos Islands Dorthe Jensen 102 Caribbean Islands Cuba Hubertus Heinicke 107 Dominica Ana Maria Martin Gonzalez 110 Essay localities 3 The Faroe Islands Kent Olsen Introduction The Faroe Islands is a treeless archipelago situated in the heart of the warm North Atlantic Current on the Wyville Thompson Ridge between 61°20’ and 62°24’ N and between 6°15’ and 7°41’ W. -

A Recent Bat Survey Reveals Bukit Barisan Selatan Landscape As A
A Recent Bat Survey Reveals Bukit Barisan Selatan Landscape as a Chiropteran Diversity Hotspot in Sumatra Author(s): Joe Chun-Chia Huang, Elly Lestari Jazdzyk, Meyner Nusalawo, Ibnu Maryanto, Maharadatunkamsi, Sigit Wiantoro, and Tigga Kingston Source: Acta Chiropterologica, 16(2):413-449. Published By: Museum and Institute of Zoology, Polish Academy of Sciences DOI: http://dx.doi.org/10.3161/150811014X687369 URL: http://www.bioone.org/doi/full/10.3161/150811014X687369 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Acta Chiropterologica, 16(2): 413–449, 2014 PL ISSN 1508-1109 © Museum and Institute of Zoology PAS doi: 10.3161/150811014X687369 A recent -

Chiroptera: Pteropodidae)
Chapter 6 Phylogenetic Relationships of Harpyionycterine Megabats (Chiroptera: Pteropodidae) NORBERTO P. GIANNINI1,2, FRANCISCA CUNHA ALMEIDA1,3, AND NANCY B. SIMMONS1 ABSTRACT After almost 70 years of stability following publication of Andersen’s (1912) monograph on the group, the systematics of megachiropteran bats (Chiroptera: Pteropodidae) was thrown into flux with the advent of molecular phylogenetics in the 1980s—a state where it has remained ever since. One particularly problematic group has been the Austromalayan Harpyionycterinae, currently thought to include Dobsonia and Harpyionycteris, and probably also Aproteles.Inthis contribution we revisit the systematics of harpyionycterines. We examine historical hypotheses of relationships including the suggestion by O. Thomas (1896) that the rousettine Boneia bidens may be related to Harpyionycteris, and report the results of a series of phylogenetic analyses based on new as well as previously published sequence data from the genes RAG1, RAG2, vWF, c-mos, cytb, 12S, tVal, 16S,andND2. Despite a striking lack of morphological synapomorphies, results of our combined analyses indicate that Boneia groups with Aproteles, Dobsonia, and Harpyionycteris in a well-supported, expanded Harpyionycterinae. While monophyly of this group is well supported, topological changes within this clade across analyses of different data partitions indicate conflicting phylogenetic signals in the mitochondrial partition. The position of the harpyionycterine clade within the megachiropteran tree remains somewhat uncertain. Nevertheless, biogeographic patterns (vicariance-dispersal events) within Harpyionycterinae appear clear and can be directly linked to major biogeographic boundaries of the Austromalayan region. The new phylogeny of Harpionycterinae also provides a new framework for interpreting aspects of dental evolution in pteropodids (e.g., reduction in the incisor dentition) and allows prediction of roosting habits for Harpyionycteris, whose habits are unknown. -
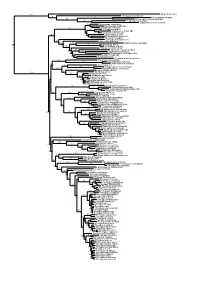
Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Ctz78-02 (02) Lee Et Al.Indd 51 14 08 2009 13:12 52 Lee Et Al
Contributions to Zoology, 78 (2) 51-64 (2009) Variation in the nocturnal foraging distribution of and resource use by endangered Ryukyu flying foxes(Pteropus dasymallus) on Iriomotejima Island, Japan Ya-Fu Lee1, 4, Tokushiro Takaso2, 5, Tzen-Yuh Chiang1, 6, Yen-Min Kuo1, 7, Nozomi Nakanishi2, 8, Hsy-Yu Tzeng3, 9, Keiko Yasuda2 1 Department of Life Sciences and Institute of Biodiversity, National Cheng Kung University, Tainan 701, Taiwan 2 The Iriomote Project, Research Institute for Humanity and Nature, 671 Iriomote, Takatomi-cho, Okinawa 907- 1542, Japan 3 Hengchun Research Center, Taiwan Forestry Research Institute, Pingtung 946, Taiwan 4 E-mail: [email protected] 5 E-mail: [email protected] 6 E-mail: [email protected] 7 E-mail: [email protected] 8 E-mail: [email protected] 9 E-mail: [email protected] Key words: abundance, bats, Chiroptera, diet, figs, frugivores, habitat Abstract Contents The nocturnal distribution and resource use by Ryukyu flying foxes Introduction ........................................................................................ 51 was studied along 28 transects, covering five types of habitats, on Material and methods ........................................................................ 53 Iriomote Island, Japan, from early June to late September, 2005. Study sites ..................................................................................... 53 Bats were mostly encountered solitarily (66.8%) or in pairs (16.8%), Bat and habitat census ................................................................ -

Priority-Setting for Philippine Bats Using Practical Approach to Guide Effective Species Conservation and Policy-Making in the Anthropocene
Published by Associazione Teriologica Italiana Volume 30 (1): 74–83, 2019 Hystrix, the Italian Journal of Mammalogy Available online at: http://www.italian-journal-of-mammalogy.it doi:10.4404/hystrix–00172-2019 Research Article Priority-setting for Philippine bats using practical approach to guide effective species conservation and policy-making in the Anthropocene Krizler Cejuela Tanalgo1,2,3,4,∗, Alice Catherine Hughes1,3 1Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 2International College, University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China 3Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 4Department of Biological Sciences, College of Arts and Sciences, University of Southern Mindanao, Kabacan 9407, North Cotabato, the Republic of the Philippines Keywords: Abstract asian tropics endemism National level approaches to the development and implementation of effective conservation policy forest loss and practice are often challenged by limited capacity and resources. Developing relevant and hunting achievable priorities at the national level is a crucial step for effective conservation. The Philip- islands pine archipelago includes over 7000 islands and is one of only two countries considered both a oil palm global biodiversity hotspot and a megadiversity country. Yet, few studies have conducted over- arching synthesis for threats and conservation priorities of any species group. As bats make up a Article history: significant proportion of mammalian diversity in the Philippines and fulfil vital roles to maintain Received: 01/19/2019 ecosystem health and services we focus on assessing the threats and priorities to their conserva- Accepted: 24/06/2019 tion across the Philippines. -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

The Philippine Flying Foxes, Acerodon Jubatus and Pteropus Vampyrus Lanensis
Journal of Mammalogy, 86(4):719- 728, 2005 DIETARY HABITS OF THE WORLD’S LARGEST BATS: THE PHILIPPINE FLYING FOXES, ACERODON JUBATUS AND PTEROPUS VAMPYRUS LANENSIS Sam C. Stier* and Tammy L. M ildenstein College of Forestry and Conservation, University of Montana, Missoula, MT 59802, USA The endemic and endangered golden- crowned flying fox (Acerodon jubatus) coroosts with the much more common and widespread giant Philippine fmit bat (Pteropus vampyrus ianensis) in lowland dipterocarp forests throughout the Philippine Islands. The number of these mixed roost- colonies and the populations of flying foxes in them have declined dramatically in the last century. We used fecal analysis, interviews of bat hunters, and personal observations to describe the dietary habits of both bat species at one of the largest mixed roosts remaining, near Subic Bay, west- central Luzon. Dietary items were deemed “important” if used consistently on a seasonal basis or throughout the year, ubiquitously throughout the population, and if they were of clear nutritional value. Of the 771 droppings examined over a 2.5 -year period (1998-2000), seeds from Ficus were predominant in the droppings of both species and met these criteria, particularly hemiepiphytic species (41% of droppings of A. jubatus) and Ficus variegata (34% of droppings of P. v. ianensis and 22% of droppings of A. jubatus). Information from bat hunter interviews expanded our knowledge of the dietary habits of both bat species, and corroborated the fecal analyses and personal observations. Results from this study suggest that A. jubatus is a forest obligate, foraging on fruits and leaves from plant species restricted to lowland, mature natural forests, particularly using a small subset of hemiepiphytic and other Ficus species throughout the year.