Color Images, Color Spaces and Color Image Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Color Management
Color Management hotoshop 5.0 was justifiably praised as a ground- breaking upgrade when it was released in the summer of 1998, although the changes made to the color P management setup were less well received in some quarters. This was because the revised system was perceived to be complex and unnecessary. Bruce Fraser once said of the Photoshop 5.0 color management system ‘it’s push-button simple, as long as you know which of the 60 or so buttons to push!’ Attitudes have changed since then (as has the interface) and it is fair to say that most people working today in the pre-press industry are now using ICC color profile managed workflows. The aim of this chapter is to introduce the basic concepts of color management before looking at the color management interface in Photoshop and the various color management settings. 1 Color management Adobe Photoshop CS6 for Photographers: www.photoshopforphotographers.com The need for color management An advertising agency art buyer was once invited to address a meeting of photographers. The chair, Mike Laye, suggested we could ask him anything we wanted, except ‘Would you like to see my book?’ And if he had already seen your book, we couldn’t ask him why he hadn’t called it back in again. And if he had called it in again we were not allowed to ask why we didn’t get the job. And finally, if we did get the job we were absolutely forbidden to ask why the color in the printed ad looked nothing like the original photograph! That in a nutshell is a problem which has bugged many of us throughout our working lives, and it is one which will be familiar to anyone who has ever experienced the difficulty of matching colors on a computer display with the original or a printed output. -

Component Video W/Audio Distribution Amplifier AT-COMP18AD
Component Video W/Audio Distribution Amplifier AT-COMP18AD User Manual Toll free: 1-877-536-3976 Local: 1-408-962-0515 www.atlona.com TABLE OF CONTENTS 1. Introduction 2 2. Features 2 3. Package Contents 2 4. Specifications 3 5. Panel Description 3 5.1. Front Panel 3 5.2. Rear Panel 3 6. Connection and Installation 4 7. Safety Information 5 8. Warranty 6 9. Atlona Product Registration 7 Toll free: 1-877-536-3976 Local: 1-408-962-0515 1 www.atlona.com INTRODUCTION The 1x8 Component w/Audio Distribution Amplifier is the perfect solution for anyone who needs to send one source of high definition component video with Audio to multiple displays at the same time. It supports all component sources and displays. Supported resolutions: 480i, 480p, 576i, 576p, 720p, 1080i and 1080p. The AT-COMP18AD is capable to send signal from one source to all 8 displays at the same time without signal degradation. The 1x8 Splitter will support long cable runs up to100ft ( 30M ). FEATURES • Supports both SD (composite, YCbCr) and HDTV (YPbPr) input signal. • Audio can be analog stereo (L/R) or digital coxial (S/PDIF) • Accepts one component input and splits it to 8 identical and buffered outputs without any loss. • When input is composite video, it can connect up to 3 different video sources and output 8 identical and buffered signal for each input source. • High bandwidth performance 650MHz at -3dB. • Ideal for presentation and home theater applications. PACKAGE CONTENT • 1:8 Component w/audio Splitter • 1 x 6 foot Component cable • 1 x 6 foot Audio cable • -

Color Models
Color Models Jian Huang CS456 Main Color Spaces • CIE XYZ, xyY • RGB, CMYK • HSV (Munsell, HSL, IHS) • Lab, UVW, YUV, YCrCb, Luv, Differences in Color Spaces • What is the use? For display, editing, computation, compression, …? • Several key (very often conflicting) features may be sought after: – Additive (RGB) or subtractive (CMYK) – Separation of luminance and chromaticity – Equal distance between colors are equally perceivable CIE Standard • CIE: International Commission on Illumination (Comission Internationale de l’Eclairage). • Human perception based standard (1931), established with color matching experiment • Standard observer: a composite of a group of 15 to 20 people CIE Experiment CIE Experiment Result • Three pure light source: R = 700 nm, G = 546 nm, B = 436 nm. CIE Color Space • 3 hypothetical light sources, X, Y, and Z, which yield positive matching curves • Y: roughly corresponds to luminous efficiency characteristic of human eye CIE Color Space CIE xyY Space • Irregular 3D volume shape is difficult to understand • Chromaticity diagram (the same color of the varying intensity, Y, should all end up at the same point) Color Gamut • The range of color representation of a display device RGB (monitors) • The de facto standard The RGB Cube • RGB color space is perceptually non-linear • RGB space is a subset of the colors human can perceive • Con: what is ‘bloody red’ in RGB? CMY(K): printing • Cyan, Magenta, Yellow (Black) – CMY(K) • A subtractive color model dye color absorbs reflects cyan red blue and green magenta green blue and red yellow blue red and green black all none RGB and CMY • Converting between RGB and CMY RGB and CMY HSV • This color model is based on polar coordinates, not Cartesian coordinates. -

Image Processing Terms Used in This Section Working with Different Screen Bit Describes How to Determine the Screen Bit Depth of Your Depths (P
13 Color This section describes the toolbox functions that help you work with color image data. Note that “color” includes shades of gray; therefore much of the discussion in this chapter applies to grayscale images as well as color images. Topics covered include Terminology (p. 13-2) Provides definitions of image processing terms used in this section Working with Different Screen Bit Describes how to determine the screen bit depth of your Depths (p. 13-3) system and provides recommendations if you can change the bit depth Reducing the Number of Colors in an Describes how to use imapprox and rgb2ind to reduce the Image (p. 13-6) number of colors in an image, including information about dithering Converting to Other Color Spaces Defines the concept of image color space and describes (p. 13-15) how to convert images between color spaces 13 Color Terminology An understanding of the following terms will help you to use this chapter. Terms Definitions Approximation The method by which the software chooses replacement colors in the event that direct matches cannot be found. The methods of approximation discussed in this chapter are colormap mapping, uniform quantization, and minimum variance quantization. Indexed image An image whose pixel values are direct indices into an RGB colormap. In MATLAB, an indexed image is represented by an array of class uint8, uint16, or double. The colormap is always an m-by-3 array of class double. We often use the variable name X to represent an indexed image in memory, and map to represent the colormap. Intensity image An image consisting of intensity (grayscale) values. -

An Improved SPSIM Index for Image Quality Assessment
S S symmetry Article An Improved SPSIM Index for Image Quality Assessment Mariusz Frackiewicz * , Grzegorz Szolc and Henryk Palus Department of Data Science and Engineering, Silesian University of Technology, Akademicka 16, 44-100 Gliwice, Poland; [email protected] (G.S.); [email protected] (H.P.) * Correspondence: [email protected]; Tel.: +48-32-2371066 Abstract: Objective image quality assessment (IQA) measures are playing an increasingly important role in the evaluation of digital image quality. New IQA indices are expected to be strongly correlated with subjective observer evaluations expressed by Mean Opinion Score (MOS) or Difference Mean Opinion Score (DMOS). One such recently proposed index is the SuperPixel-based SIMilarity (SPSIM) index, which uses superpixel patches instead of a rectangular pixel grid. The authors of this paper have proposed three modifications to the SPSIM index. For this purpose, the color space used by SPSIM was changed and the way SPSIM determines similarity maps was modified using methods derived from an algorithm for computing the Mean Deviation Similarity Index (MDSI). The third modification was a combination of the first two. These three new quality indices were used in the assessment process. The experimental results obtained for many color images from five image databases demonstrated the advantages of the proposed SPSIM modifications. Keywords: image quality assessment; image databases; superpixels; color image; color space; image quality measures Citation: Frackiewicz, M.; Szolc, G.; Palus, H. An Improved SPSIM Index 1. Introduction for Image Quality Assessment. Quantitative domination of acquired color images over gray level images results in Symmetry 2021, 13, 518. https:// the development not only of color image processing methods but also of Image Quality doi.org/10.3390/sym13030518 Assessment (IQA) methods. -

COLOR SPACE MODELS for VIDEO and CHROMA SUBSAMPLING
COLOR SPACE MODELS for VIDEO and CHROMA SUBSAMPLING Color space A color model is an abstract mathematical model describing the way colors can be represented as tuples of numbers, typically as three or four values or color components (e.g. RGB and CMYK are color models). However, a color model with no associated mapping function to an absolute color space is a more or less arbitrary color system with little connection to the requirements of any given application. Adding a certain mapping function between the color model and a certain reference color space results in a definite "footprint" within the reference color space. This "footprint" is known as a gamut, and, in combination with the color model, defines a new color space. For example, Adobe RGB and sRGB are two different absolute color spaces, both based on the RGB model. In the most generic sense of the definition above, color spaces can be defined without the use of a color model. These spaces, such as Pantone, are in effect a given set of names or numbers which are defined by the existence of a corresponding set of physical color swatches. This article focuses on the mathematical model concept. Understanding the concept Most people have heard that a wide range of colors can be created by the primary colors red, blue, and yellow, if working with paints. Those colors then define a color space. We can specify the amount of red color as the X axis, the amount of blue as the Y axis, and the amount of yellow as the Z axis, giving us a three-dimensional space, wherein every possible color has a unique position. -
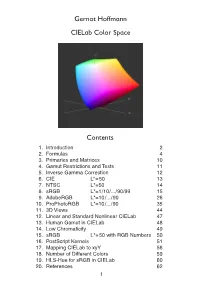
Cielab Color Space
Gernot Hoffmann CIELab Color Space Contents . Introduction 2 2. Formulas 4 3. Primaries and Matrices 0 4. Gamut Restrictions and Tests 5. Inverse Gamma Correction 2 6. CIE L*=50 3 7. NTSC L*=50 4 8. sRGB L*=/0/.../90/99 5 9. AdobeRGB L*=0/.../90 26 0. ProPhotoRGB L*=0/.../90 35 . 3D Views 44 2. Linear and Standard Nonlinear CIELab 47 3. Human Gamut in CIELab 48 4. Low Chromaticity 49 5. sRGB L*=50 with RGB Numbers 50 6. PostScript Kernels 5 7. Mapping CIELab to xyY 56 8. Number of Different Colors 59 9. HLS-Hue for sRGB in CIELab 60 20. References 62 1.1 Introduction CIE XYZ is an absolute color space (not device dependent). Each visible color has non-negative coordinates X,Y,Z. CIE xyY, the horseshoe diagram as shown below, is a perspective projection of XYZ coordinates onto a plane xy. The luminance is missing. CIELab is a nonlinear transformation of XYZ into coordinates L*,a*,b*. The gamut for any RGB color system is a triangle in the CIE xyY chromaticity diagram, here shown for the CIE primaries, the NTSC primaries, the Rec.709 primaries (which are also valid for sRGB and therefore for many PC monitors) and the non-physical working space ProPhotoRGB. The white points are individually defined for the color spaces. The CIELab color space was intended for equal perceptual differences for equal chan- ges in the coordinates L*,a* and b*. Color differences deltaE are defined as Euclidian distances in CIELab. This document shows color charts in CIELab for several RGB color spaces. -

Painting Part 3 1
.T 720 (07) | 157 ) v.10 • pt. 3 I n I I I 6 International Correspondence Schools, Scranton, Pa. Painting By DURWARD E. NICHOLSON Technical Writer, International Correspondence Schools and DAVID T. JONES, B.Arch. Director, School of Architecture and the Building Trades International Correspondence Schools 6227C Part 3 Edition 1 International Correspondence Schools, Scranton, Pennsylvania International Correspondence Schools, Canadian, Ltd., Montreal, Canadc Painting \A jo Pa3RT 3 “I find in life lliat most affairs tliat require serious handling are distasteful. For this reason, I have always believed that the successful man has the hardest battle with himself rather than with the other fellow'. By To bring one’s self to a frame of mind and to the proper energy to accomplish things that require plain DURWARD E. NICHOLSON hard work continuously is the one big battle that Technical Writer everyone has. When this battle is won for all time, then everything is easy.” \ International Correspondence Schools —Thomas A. Buckner and DAVID T. JONES, B. Arch. 33 Director, School of Architecture and the Building Trades International Correspondence Schools B Member, American Institute of Architects Member, Construction Specifications Institute Serial 6227C © 1981 by INTERNATIONAL TEXTBOOK COMPANY Printed in the United States of America All rights reserved International Correspondence Schools > Scranton, Pennsylvania\/ International Correspondence Schools Canadian, Ltd. ICS Montreal, Canada ▼ O (7M V\) v-V*- / / *r? 1 \/> IO What This Text Covers . /’ v- 3 Painting Part 3 1. PlGX OLORS ___________________ ________ Pages 1 to 14 The color of paint depends on the colors of the pigments that are mixed with the vehicle. -

Real-Time Supervised Detection of Pink Areas in Dermoscopic Images of Melanoma: Importance of Color Shades, Texture and Location
Missouri University of Science and Technology Scholars' Mine Electrical and Computer Engineering Faculty Research & Creative Works Electrical and Computer Engineering 01 Nov 2015 Real-Time Supervised Detection of Pink Areas in Dermoscopic Images of Melanoma: Importance of Color Shades, Texture and Location Ravneet Kaur P. P. Albano Justin G. Cole Jason R. Hagerty et. al. For a complete list of authors, see https://scholarsmine.mst.edu/ele_comeng_facwork/3045 Follow this and additional works at: https://scholarsmine.mst.edu/ele_comeng_facwork Part of the Chemistry Commons, and the Electrical and Computer Engineering Commons Recommended Citation R. Kaur et al., "Real-Time Supervised Detection of Pink Areas in Dermoscopic Images of Melanoma: Importance of Color Shades, Texture and Location," Skin Research and Technology, vol. 21, no. 4, pp. 466-473, John Wiley & Sons, Nov 2015. The definitive version is available at https://doi.org/10.1111/srt.12216 This Article - Journal is brought to you for free and open access by Scholars' Mine. It has been accepted for inclusion in Electrical and Computer Engineering Faculty Research & Creative Works by an authorized administrator of Scholars' Mine. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. Published in final edited form as: Skin Res Technol. 2015 November ; 21(4): 466–473. doi:10.1111/srt.12216. Real-time Supervised Detection of Pink Areas in Dermoscopic Images of Melanoma: Importance of Color Shades, Texture and Location Ravneet Kaur, MS, Department of Electrical and Computer Engineering, Southern Illinois University Edwardsville, Campus Box 1801, Edwardsville, IL 62026-1801, Telephone: 618-210-6223, [email protected] Peter P. -

NEC Multisync® PA311D Wide Gamut Color Critical Display Designed for Photography and Video Production
NEC MultiSync® PA311D Wide gamut color critical display designed for photography and video production 1419058943 High resolution and incredible, predictable color accuracy. The 31” MultiSync PA311D is the ultimate desktop display for applications where precise color is essential. The innovative wide-gamut LED backlight provides 100% coverage of Adobe RGB color space and 98% coverage of DCI-P3, enabling more accurate colors to be displayed on screen. Utilizing a high performance IPS LCD panel and backed by a 4 year warranty with Advanced Exchange, the MultiSync PA311D delivers high quality, accurate images simply and beautifully. Impeccable Image Performance The wide-gamut LED LCD backlight combined with NEC’s exclusive SpectraView Engine deliver precise color in every environment. • True 4K resolution (4096 x 2160) offers a high pixel density • Up to 100% coverage of Adobe RGB color space and 98% coverage of DCI-P3 • 10-bit HDMI and DisplayPort inputs display up to 1.07 billion colors out of a palette of 4.3 trillion colors Ultimate Color Management The sophisticated SpectraView Engine provides extensive, intuitive control over color settings. • MultiProfiler software and on-screen controls provide access to thousands of color gamut, gamma, white point, brightness and contrast combinations • Internal 14-bit 3D lookup tables (LUTs) work with optional SpectraViewII color calibration solution for unparalleled color accuracy A Perfect Fit for Your Workspace A Better Workflow Future-proof connectivity, great ergonomics, and VESA mount Exclusive, -

Accurately Reproducing Pantone Colors on Digital Presses
Accurately Reproducing Pantone Colors on Digital Presses By Anne Howard Graphic Communication Department College of Liberal Arts California Polytechnic State University June 2012 Abstract Anne Howard Graphic Communication Department, June 2012 Advisor: Dr. Xiaoying Rong The purpose of this study was to find out how accurately digital presses reproduce Pantone spot colors. The Pantone Matching System is a printing industry standard for spot colors. Because digital printing is becoming more popular, this study was intended to help designers decide on whether they should print Pantone colors on digital presses and expect to see similar colors on paper as they do on a computer monitor. This study investigated how a Xerox DocuColor 2060, Ricoh Pro C900s, and a Konica Minolta bizhub Press C8000 with default settings could print 45 Pantone colors from the Uncoated Solid color book with only the use of cyan, magenta, yellow and black toner. After creating a profile with a GRACoL target sheet, the 45 colors were printed again, measured and compared to the original Pantone Swatch book. Results from this study showed that the profile helped correct the DocuColor color output, however, the Konica Minolta and Ricoh color outputs generally produced the same as they did without the profile. The Konica Minolta and Ricoh have much newer versions of the EFI Fiery RIPs than the DocuColor so they are more likely to interpret Pantone colors the same way as when a profile is used. If printers are using newer presses, they should expect to see consistent color output of Pantone colors with or without profiles when using default settings. -

Image Processing
IMAGE PROCESSING ROBOTICS CLUB SUMMER CAMP’12 WHAT IS IMAGE PROCESSING? IMAGE PROCESSING = IMAGE + PROCESSING WHAT IS IMAGE? IMAGE = Made up of PIXELS Each Pixels is like an array of Numbers. Numbers determine colour of Pixel. TYPES OF IMAGES 1. BINARY IMAGE 2. GREYSCALE IMAGE 3. COLOURED IMAGE BINARY IMAGE Each Pixel has either 1 (White) or 0 (Black) Depth =1 (bit) Number of Channels = 1 0 0 0 0 0 0 0 0 0 0 0 1 1 1 1 1 0 0 0 0 1 1 1 1 1 0 0 0 0 1 1 1 1 1 0 0 0 0 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 GRAYSCALE Each Pixel has a value from 0 to 255. 0 : black and 1 : White Between 0 and 255 are shades of b&w. Depth=8 (bits) Number of Channels =1 GRAYSCALE IMAGE RGB IMAGE Each Pixel stores 3 values :- R : 0- 255 G: 0 -255 B : 0-255 Depth=8 (bits) Number of Channels = 3 RGB IMAGE HSV IMAGE Each pixel stores 3 values :- H ( hue ) : 0 -180 S (saturation) : 0-255 V (value) : 0-255 Depth = 8 (bits) Number of Channels = 3 Note : Hue in general is from 0-360 , but as hue is 8 bits in OpenCV , it is shrinked to 180 STARTING WITH OPENCV OpenCV is a library for C language developed for Image Processing To embed opencv library in Dev C complier , follow instructions in :- http://opencv.willowgarage.com/wiki/DevCpp HEADER FILES IN C After embedding openCV library in Dev C include following header files:- #include "cv.h" #include "highgui.h" IMAGE POINTER An image is stored as a structure IplImage with following elements :- int height Width int width int nChannels int depth Height char *imageData int widthStep ….